硫化物生成对铁水岩地球化学及相关砷归宿的影响
IF 7.6
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
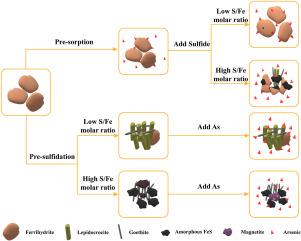
地下水中砷(As)的行为与铁水物的硫化密切相关。在无水铁-砷-硫化物三元系统中,无水铁要么在硫化物还原之前吸附砷,要么首先遇到硫化物,然后与水体中的砷相互作用,改变砷的命运。然而,人们对它们在铁水物矿物学转变中的相对作用以及随后相关的砷迁移/再分布仍知之甚少。因此,我们进行了结合化学、显微镜和光谱分析的分批实验,以澄清铁酸盐的地球化学及其对砷行为的影响。结果表明,在预吸附组中,由于 As 的延迟效应,次生矿物主要以无定形相存在。在硫化物浓度较低(S/Fe=0.04)时,残余铁水物的含量较大,有利于砷的固定。但在硫化物浓度较高(S/Fe=0.8)时,As 最初释放到溶液中,随后被次生矿物重新固定。次生矿物对 As 的吸附能力随着无定形麦饭石形成的增加而降低。在硫化前组别中,铁酸盐的快速还原促进了结晶矿物的形成,大大降低了它们的吸附能力。在硫化物浓度较低时,释放的砷部分被吸附在结晶鹅绿泥石和鳞绿泥石的表面。在硫化物浓度较高的情况下,磁铁矿形成,并通过融入磁铁矿颗粒而有利于砷的固定。这些结果为了解地下水系统中铁、硒和砷的地球化学提供了重要依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effect of sulfidogenesis on ferrihydrite geochemistry and associated arsenic fate
The behavior of arsenic (As) in groundwater is closely related to the sulfidation of ferrihydrite. In the ternary ferrihydrite-As-sulfide system, ferrihydrite can either initially adsorb As before sulfide reduction or first encounter sulfide and then interact with the aqueous As, altering As fate. However, their relative contributions to the mineralogical transformation of ferrihydrite and subsequently associated As mobilization/redistribution remain poorly understood. Therefore, batch experiments combined with chemical, microscopic, and spectroscopic analyses were conducted to clarify the geochemistry of ferrihydrite and its influence on As behavior. Results indicated that in the pre-sorption groups, the secondary minerals were predominantly presented in amorphous phase due to the retardative effect of As. At low sulfide concentrations (S/Fe = 0.04), the content of residual ferrihydrite was large, which favored As immobilization. At high sulfide concentrations (S/Fe = 0.8), however, As was initially released into the solution and subsequently re-immobilized by secondary minerals. The adsorption capacity of the secondary minerals for As decreased with the increase in amorphous mackinawite formation. In the pre-sulfidation groups, rapid ferrihydrite reduction promoted the formation of crystalline minerals, significantly reducing their adsorption capacity. At low sulfide concentrations, the released As was partially adsorbed on the surface of crystalline goethite and lepidocrocite. At high sulfide concentrations, magnetite formed and favored As immobilization through its incorporation into magnetite particles. These results provide important insights into the geochemistry of Fe, S, and As in groundwater systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


