PNPLA3(148M) 是一种功能增益突变,可通过抑制 ATGL 介导的甘油三酯水解促进肝脂肪变性
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
背景& 目的PNPLA3(148M)(类磷脂酶域含蛋白 3)是脂肪性肝病(SLD)最具影响力的遗传风险因素。一个尚未解决的关键问题是,PNPLA3(148M)是功能缺失还是功能增益。在这里,我们检验了 PNPLA3 通过封存 ATGL(脂肪 TG 脂肪酶)的辅助因子 ABHD5(含α/β水解酶结构域的蛋白 5)从而限制肝脏甘油三酯(TG)的动员来导致脂肪肝的假设。从人体细胞中纯化的重组蛋白被用来比较 PNPLA3 和 ATGL 在 ABHD5 存在或不存在时的 TG 水解活性。用腺病毒和腺相关病毒分别在肝脏特异性 Atgl-/- 小鼠体内表达 PNPLA3 和在 Pnpla3M/M 小鼠肝脏中表达 ABHD5。ABHD5 与 PNPLA3(WT)和 PNPLA3(148M)之间的相互作用强度没有差异。与之前的研究结果不同,我们发现 PNPLA3 与 ATGL 一样,在使用纯化蛋白的体外试验中被 ABHD5 激活。与 PNPLA3(148M) 相关的 TG 水解抑制需要 ATGL 的表达以及 PNPLA3 位于 LD 上。最后,ABHD5 的过表达逆转了 Pnpla3M/M 小鼠的肝脏脂肪变性。结论这些研究结果支持这样一个前提,即 PNPLA3(148M) 是一种功能增益突变,它通过积聚在 LD 上并以 ABHD5 依赖性方式抑制 ATGL 介导的脂肪分解,从而促进肝脏脂肪变性。我们的研究结果预测,减少而不是增加PNPLA3的表达将是治疗PNPLA3(148M)相关SLD的最佳策略。影响和意义脂肪性肝病(SLD)是一种常见的复杂疾病,与环境和遗传风险因素有关。PNPLA3(148M)是SLD最具影响力的遗传风险因素,但其致病机制仍存在争议。我们在此提供的证据表明,PNPLA3(148M)通过封存 ABHD5 促进甘油三酯(TG)的积累,从而限制其激活 ATGL 的可用性。虽然用蛋氨酸取代异亮氨酸会降低 PNPLA3 的甘油三酯水解酶活性,但酶功能的丧失与变体的脂肪变性效应并无直接关系。正是由此导致的 PNPLA3 在 LD 上的积累干扰了 ATGL 介导的 TG 水解,从而产生了功能增益。这些发现对设计基于 PNPLA3(148M)的潜在疗法具有重要意义。降低而非增加 PNPLA3 水平有望逆转易感人群的脂肪变性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

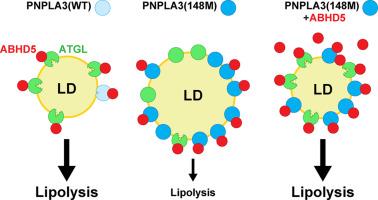
PNPLA3(148M) is a gain-of-function mutation that promotes hepatic steatosis by inhibiting ATGL-mediated triglyceride hydrolysis
Background & Aims
PNPLA3(148M) (patatin-like phospholipase domain-containing protein 3) is the most impactful genetic risk factor for steatotic liver disease. A key unresolved issue is whether PNPLA3(148M) confers a loss- or gain-of-function. Here we test the hypothesis that PNPLA3 causes steatosis by sequestering ABHD5 (α/β hydrolase domain-containing protein 5), the cofactor of ATGL (adipose TG lipase), thus limiting mobilization of hepatic triglyceride (TG).
Methods
We quantified and compared the physical interactions between ABHD5 and PNPLA3/ATGL in cultured hepatocytes using NanoBiT complementation assays and immunocytochemistry. Recombinant proteins purified from human cells were used to compare TG hydrolytic activities of PNPLA3 and ATGL in the presence or absence of ABHD5. Adenoviruses and adeno-associated viruses were used to express PNPLA3 in liver-specific Atgl-/- mice and to express ABHD5 in livers of Pnpla3M/M mice, respectively.
Results
ABHD5 interacted preferentially with PNPLA3 relative to ATGL in cultured hepatocytes. No differences were seen in the strength of the interactions between ABHD5 with PNPLA3(WT) and PNPLA3(148M). In contrast to prior findings, we found that PNPLA3, like ATGL, is activated by ABHD5 in in vitro assays using purified proteins. PNPLA3(148M)-associated inhibition of TG hydrolysis required that ATGL be expressed and that PNPLA3 be located on lipid droplets. Finally, overexpression of ABHD5 reversed the hepatic steatosis in Pnpla3M/M mice.
Conclusions
These findings support the premise that PNPLA3(148M) is a gain-of-function mutation that promotes hepatic steatosis by accumulating on lipid droplets and inhibiting ATGL-mediated lipolysis in an ABHD5-dependent manner. Our results predict that reducing, rather than increasing, PNPLA3 expression will be the best strategy to treat PNPLA3(148M)-associated steatotic liver disease.
Impact and implications
Steatotic liver disease (SLD) is a common complex disorder associated with both environmental and genetic risk factors. PNPLA3(148M) is the most impactful genetic risk factor for SLD and yet its pathogenic mechanism remains controversial. Herein, we provide evidence that PNPLA3(148M) promotes triglyceride (TG) accumulation by sequestering ABHD5, thus limiting its availability to activate ATGL. Although the substitution of methionine for isoleucine reduces the TG hydrolase activity of PNPLA3, the loss of enzymatic function is not directly related to the steatotic effect of the variant. It is the resulting accumulation of PNPLA3 on LDs that confers a gain-of-function by interfering with ATGL-mediated TG hydrolysis. These findings have implications for the design of potential PNPLA3(148M)-based therapies. Reducing, rather than increasing, PNPLA3 levels is predicted to reverse steatosis in susceptible individuals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


