酰基氟化物和烷基硅烷的双 N-杂环羰基/光氧化催化偶联反应
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
N-heterocyclic carbene(NHC)有机催化与光化学活化的结合正日益成为一种在温和、实用的条件下进行有机自由基反应的方法。作为比较容易制备和处理的有机化合物,烷基硅烷是具有吸引力的自由基化学底物,因为众所周知,相应自由基阳离子的脱硅介解速度很快。在此,我们报告了苄基硅烷衍生物作为烷基自由基源在 NHC/光氧催化的自由基与酰氟的双自由基偶联反应中的成功应用。电子相对丰富的苄基硅烷能顺利地与相应的酮类发生反应,且产率普遍较高,而对 NHC 和光催化剂的优化则扩大了反应范围,包括初级苄基底物。此外,初步实验表明,在这些条件下,含有 N、O 和 S 杂原子的有机硅烷也可以作为烷基自由基源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
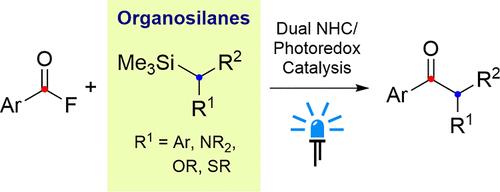
Dual N-Heterocyclic Carbene/Photoredox-Catalyzed Coupling of Acyl Fluorides and Alkyl Silanes
The combination of N-heterocyclic carbene (NHC) organocatalysis with photochemical activation is becoming increasingly established as an approach for conducting radical organic reactions under mild and practical conditions. As comparatively easy to prepare and handle organic compounds, alkyl silanes are attractive substrates for radical chemistry as desilylative mesolysis of the corresponding radical cations is known to be rapid. Here, we report the successful application of benzyl silane derivatives as source of alkyl radicals in dual NHC/photoredox-catalyzed radical–radical coupling reactions with acyl fluorides. Relatively electron-rich benzyl silanes reacted smoothly to afford the corresponding ketones in generally good yields, while optimization of the NHC and photocatalyst allowed for a wider scope including primary benzyl substrates. Furthermore, initial experiments revealed that organosilanes bearing N-, O- and S-heteroatoms can also serve as alkyl radical sources under these conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


