锰催化不对称氢化用于异丁烯酮 N-氧化物的逆选择动态动力学解析
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究揭示了一种通过锰催化的不对称氢化反应对配置易失性杂芳基酮 N-氧化物进行异选择性动态动力学解析的方法。通过使用结构上经过精细调整的手性二茂铁 P,N,N-配体,氢化反应在温和条件下顺利进行,并同时具有中心和轴向手性,从而得到多种具有手性醇结构的异构体 1-芳基异喹啉和 2-芳基吡啶 N-氧化物,并具有很高的非对映和对映选择性。氢化产物的非对映异构体可以通过三忍反应以立体特异性的方式制备,中心手性完全反转。在苯甲醛与烯丙基三氯硅烷的不对称烯丙基化反应中,这种中心和轴向手性杂芳基 N-氧化物支架作为手性催化剂的成功应用初步证明了它的价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
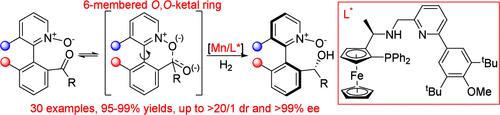
Manganese-Catalyzed Asymmetric Hydrogenation for Atroposelective Dynamic Kinetic Resolution of Heterobiaryl Ketone N-Oxides
An atroposelective dynamic kinetic resolution of configurationally labile heterobiaryl ketone N-oxides via Mn-catalyzed asymmetric hydrogenation has been disclosed. By use of a structurally finely tuned chiral ferrocenyl P,N,N-ligand, the hydrogenation proceeds smoothly under mild conditions with simultaneous installation of central and axial chirality, giving a wide range of atropisomeric 1-arylisoquinoline and 2-arylpyridine N-oxides bearing a chiral alcohol structure with high diastereo- and enantioselectivities. The diastereomer of the hydrogenation product could be readily prepared in a stereospecific way with the complete inversion of the central chirality via Mitsunobu reaction. The value of this central- and axial-chiral heterobiaryl N-oxide scaffold is preliminarily demonstrated by its successful utility as a chiral catalyst in asymmetric allylation of benzaldehyde with allyltrichlorosilane.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


