在碘化物诱导的 Cuδ+-Cu0 位点上将乙炔选择性电还原为 1,3-丁二烯
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
创建可持续化学工业的一项关键任务是使生产高附加值分子的化学过程电气化。1,3-丁二烯(1,3-BD)就是这样一种分子,它是制造合成橡胶的原料。传统上,1,3-丁二烯是在高能耗的石脑油蒸汽裂解制乙烯过程中产生的副产品。在这里,我们介绍一种从乙炔(e-C2H2R)电还原中选择性生产 1,3-BD的替代方法。通过使用碘化钾电解质,我们在 Cu2O 纳米管衍生催化剂上创建了 Cuδ+-Cu0 位点,这些位点可有效促进 e-C2H2R 生成 1,3-BD。与标准氢电极(SHE)相比,在-0.85 V电压下,生成 1,3-BD的法拉第效率达到 93%;与标准氢电极(SHE)相比,在-1.0 V电压下,生成 1,3-BD的部分电流密度为-75 mA cm-2。密度泛函理论计算表明,I-保留了 Cuδ+-Cu0 位点,这有利于乙炔的结合,从而通过 *C2H3 分子的耦合形成 1,3-BD。本文章由计算机程序翻译,如有差异,请以英文原文为准。
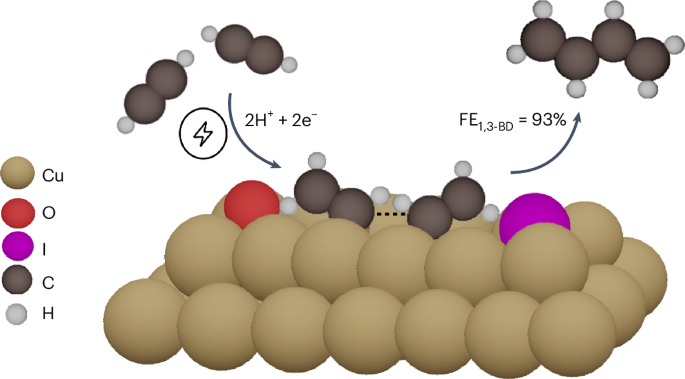

Selective electroreduction of acetylene to 1,3-butadiene on iodide-induced Cuδ+–Cu0 sites
A crucial task towards creating a sustainable chemical industry is the electrification of chemical processes that produce value-added molecules. One such molecule is 1,3-butadiene (1,3-BD), the feedstock used for manufacturing synthetic rubber. 1,3-BD is traditionally derived, as a by-product, during the energy-intensive steam cracking of naphtha to ethylene. Here we introduce an alternative approach to selectively produce 1,3-BD from the electroreduction of acetylene (e-C2H2R). By using a potassium iodide electrolyte, we created Cuδ+–Cu0 sites on a Cu2O-nanocube-derived catalyst, which are efficacious for promoting e-C2H2R to 1,3-BD. 1,3-BD was formed with a Faradaic efficiency reaching 93% at −0.85 V versus standard hydrogen electrode (SHE) and a partial current density of −75 mA cm−2 at −1.0 V versus SHE. Density functional theory calculations show that I− preserves Cuδ+–Cu0 sites, which facilitate the favourable binding of acetylene, leading to 1,3-BD formation through the coupling of *C2H3 moieties. Electrifying energy-intensive processes is a promising approach for decarbonization. Now, 1,3-butadiene is electrochemically produced from acetylene on I−−induced Cuδ+–Cu0 sites with a Faradaic efficiency of over 90% at −0.85 VSHE and a partial current density of −75 mA cm−2 at −1.0 VSHE.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


