一种级联 X 射线能量转换方法,用于放射后辉光癌症疗法
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
利用 X 射线启动长时间发光(放射余辉)并刺激光学制剂产生放射动力 1O2 为在光线无法到达的组织深度进行诊断和治疗提供了机会。然而,X 射线响应型有机发光材料因其固有的低 X 射线转换效率而十分罕见。在此,我们报告了一种级联 X 射线能量转换方法,以开发用于癌症治疗的有机放射余辉纳米探针(RANPs)。RANPs 由一个辐射波吸收器组成,该吸收器能向下转换 X 射线能量,从而发出辐射光,辐射光转移到辐射增敏剂上,产生单线态氧(1O2)。然后,1O2 与放射余辉基质反应生成活性中间体,活性中间体同时分解并发出放射余辉。通过对这种级联、粒子内放射发光能量转移和 1O2 转移过程进行微调,RANPs 具有可调波长和长半衰期,可在组织深度达 15 厘米处产生放射余辉和 1O2。此外,我们还开发了一种可激活生物标记物的纳米探针(tRANP),它能产生肿瘤特异性的放射余辉信号,从而实现超灵敏检测,并能在 X 射线剂量比无机材料低 20 倍的情况下,对微小肿瘤(1 立方毫米)进行手术切除。tRANP 的高效放射动力 1O2 生成允许以低于临床放疗的 X 射线剂量和比大多数现有无机制剂低一到两个数量级的药物剂量彻底根除肿瘤,从而延长生存率并最大限度地减少与辐射相关的不良反应。因此,我们的工作揭示了一种解决有机放射治疗材料缺乏问题的通用方法,并为实现精准癌症放射治疗提供了分子设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。

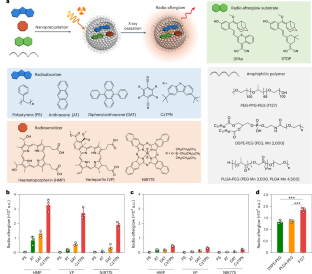
A cascade X-ray energy converting approach toward radio-afterglow cancer theranostics
Leveraging X-rays to initiate prolonged luminescence (radio-afterglow) and stimulate radiodynamic 1O2 production from optical agents provides opportunities for diagnosis and therapy at tissue depths inaccessible to light. However, X-ray-responsive organic luminescent materials are rare due to their intrinsic low X-ray conversion efficiency. Here we report a cascade X-ray energy converting approach to develop organic radio-afterglow nanoprobes (RANPs) for cancer theranostics. RANPs comprise a radiowave absorber that down-converts X-ray energy to emit radioluminescence, which is transferred to a radiosensitizer to produce singlet oxygen (1O2). 1O2 then reacts with a radio-afterglow substrate to generate an active intermediate that simultaneously decomposes to emit radio-afterglow. Through finetuning such a cascade, intraparticle radioluminescence energy transfer and the 1O2 transfer process, RANPs possess tunable wavelengths and long half-lives, and generate radio-afterglow and 1O2 at tissue depths of up to 15 cm. Moreover, we developed a biomarker-activatable nanoprobe (tRANP) that produces a tumour-specific radio-afterglow signal, leading to ultrasensitive detection and the possibility of surgical removal of diminutive tumours (1 mm3) under an X-ray dosage 20 times lower than inorganic materials. The efficient radiodynamic 1O2 generation of tRANP permits complete tumour eradication at an X-ray dosage lower than clinical radiotherapy and a drug dosage one to two orders of magnitude lower than most existing inorganic agents, leading to prolonged survival rates with minimized radiation-related adverse effects. Thus, our work reveals a generic approach to address the lack of organic radiotheranostic materials and provides molecular design towards precision cancer radiotherapy. Here, the authors present the design, molecular assembly and mechanistic insights into an organic, biomarker-activatable radiotheranostic nanoprobe for image-guided precision cancer radiotherapy with sensitivity up to a depth of 15 cm in thick tissues.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


