膳食缺锌通过 IL-13 促进小鼠鲍曼不动杆菌肺部感染
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
膳食缺锌是肺炎的一个主要风险因素。鲍曼不动杆菌是导致呼吸机相关性肺炎的主要病因,由于对多种药物的耐药性不断增加,它已成为一种严重的公共卫生威胁。鲍曼不动杆菌肺炎的高危人群缺锌的风险也在增加。在这里,我们建立了一个饮食缺锌和急性鲍曼不动杆菌肺炎的小鼠模型,以验证宿主缺锌导致鲍曼不动杆菌发病的假设。我们发现,缺锌小鼠肺部的鲍曼尼菌负荷明显增加,并向脾脏播散,死亡率较高。在感染过程中,缺锌小鼠会产生更多的促炎细胞因子,包括IL-13。服用IL-13会促进鲍曼尼氏菌在缺锌小鼠体内的扩散,而抗体中和IL-13则能保护缺锌小鼠在感染期间免受鲍曼尼氏菌的扩散和死亡。这些数据凸显了抗IL-13抗体疗法治疗肺炎的潜力,这种疗法在人体中耐受性良好。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dietary zinc deficiency promotes Acinetobacter baumannii lung infection via IL-13 in mice
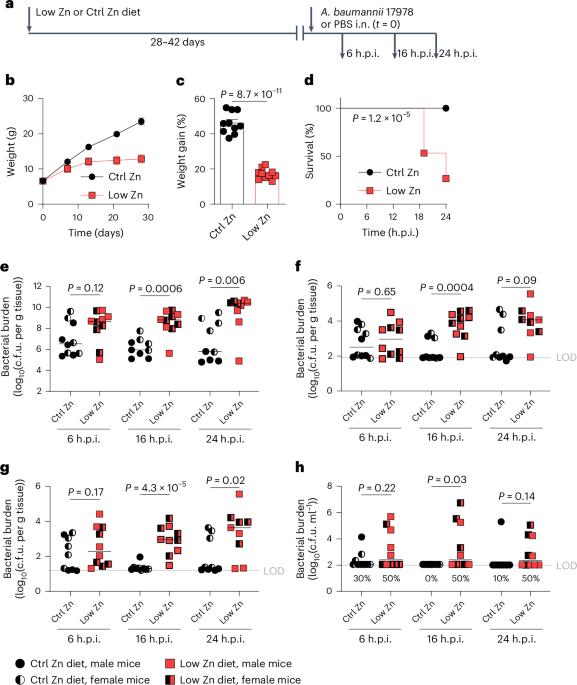
Dietary zinc deficiency is a major risk factor for pneumonia. Acinetobacter baumannii is a leading cause of ventilator-associated pneumonia and a critical public health threat due to increasing rates of multidrug resistance. Patient populations at increased risk for A. baumannii pneumonia are also at increased risk of zinc deficiency. Here we established a mouse model of dietary zinc deficiency and acute A. baumannii pneumonia to test the hypothesis that host zinc deficiency contributes to A. baumannii pathogenesis. We showed that zinc-deficient mice have significantly increased A. baumannii burdens in the lungs, dissemination to the spleen and higher mortality. During infection, zinc-deficient mice produce more pro-inflammatory cytokines, including IL-13. Administration of IL-13 promotes A. baumannii dissemination in zinc-sufficient mice, while antibody neutralization of IL-13 protects zinc-deficient mice from A. baumannii dissemination and mortality during infection. These data highlight the therapeutic potential of anti-IL-13 antibody treatments, which are well tolerated in humans, for the treatment of pneumonia. Increased IL-13 drives increased bacterial dissemination and mortality following Acinetobacter baumannii lung infection of zinc-deficient mice and can be countered by anti-IL-13 antibody therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


