受损肠隐窝中的染色质重塑可协调冗余的 TGFβ 和 Hippo 信号,从而推动肠道再生
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
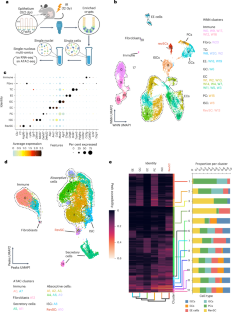
成功组织再生的细胞状态动力学特征尚不明确。在肠道中,损伤会促使上皮细胞重编程为复兴干细胞(revSCs),重建Lgr5+肠干细胞(ISCs)。这里对辐照后再生的小鼠隐窝进行的单核多组学研究显示,revSC染色质可及性与ISC和分化系重叠。虽然revSC基因本身在整个平衡上皮细胞中都可获得,但损伤诱导的隐窝染色质重塑汇聚于Hippo和转化生长因子-β(TGFβ)信号通路,我们发现TGFβ信号通路被短暂激活并直接诱导功能性revSC。组合基因表达分析进一步表明了revSCs的多种来源,我们还证明了TGFβ可将肠细胞、鹅口疮细胞和paneth细胞重编程为revSCs,并显示单个revSCs可形成器官组织。尽管如此,TGFβ 信号的缺失会导致轻微的再生缺陷,而干扰 Hippo 和 TGFβ 则会导致严重的缺陷和死亡。因此,肠道再生已准备就绪,可通过隐窝定位的瞬时形态发生器的补偿系统激活,从而支持上皮重编程和强大的肠道修复。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Chromatin remodelling in damaged intestinal crypts orchestrates redundant TGFβ and Hippo signalling to drive regeneration
Cell state dynamics underlying successful tissue regeneration are undercharacterized. In the intestine, damage prompts epithelial reprogramming into revival stem cells (revSCs) that reconstitute Lgr5+ intestinal stem cells (ISCs). Here single-nuclear multi-omics of mouse crypts regenerating from irradiation shows revSC chromatin accessibility overlaps with ISCs and differentiated lineages. While revSC genes themselves are accessible throughout homeostatic epithelia, damage-induced remodelling of chromatin in the crypt converges on Hippo and the transforming growth factor-beta (TGFβ) signalling pathway, which we show is transiently activated and directly induces functional revSCs. Combinatorial gene expression analysis further suggests multiple sources of revSCs, and we demonstrate TGFβ can reprogramme enterocytes, goblet and paneth cells into revSCs and show individual revSCs form organoids. Despite this, loss of TGFβ signalling yields mild regenerative defects, whereas interference in both Hippo and TGFβ leads to profound defects and death. Intestinal regeneration is thus poised for activation by a compensatory system of crypt-localized, transient morphogen cues that support epithelial reprogramming and robust intestinal repair. Using deep single-nucleus multi-omics profiling, Fink et al. report transition states between crypt epithelial cells and a revival stem cell lineage. They find that the TGFβ and Hippo signalling pathways cooperatively drive intestinal regeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


