塞马鲁肽通过增强获得性抗肿瘤免疫力来减缓乳腺癌的生长和恶化。
IF 6.9
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
塞马鲁肽是一种胰高血糖素样肽 1 受体激动剂,是一种抗糖尿病药物,最近显示出良好的免疫调节和抗肿瘤效果。乳腺癌是全球女性最常见的癌症类型。本研究的目的是分析semaglutide对4T1小鼠乳腺癌模型抗肿瘤免疫的影响。诱发乳腺癌后,BALB/C小鼠腹腔注射塞马鲁肽。服用塞马鲁肽可减缓肿瘤的出现、生长和恶化。这种抗糖尿病药物在体外既没有直接的细胞毒性作用,也没有血管生成作用。此外,消耗 NK 细胞对接受过塞马鲁肽治疗的小鼠的肿瘤生长没有影响,这表明了一种非 NK 细胞依赖性机制。但塞马鲁肽增加了CD11c+树突状细胞的积累和成熟,同时降低了脾脏和原发性肿瘤中FoxP3+调节性T细胞的比例。此外,塞马鲁肽还能增加肿瘤浸润,促进体内T细胞的抗肿瘤表型。此外,塞马鲁肽还能增强体外CD8+ T细胞的细胞毒性能力。这些结果表明,塞马鲁肽能增强获得性抗肿瘤免疫反应,未来有望用于恶性肿瘤的治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
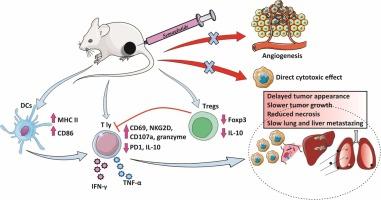
Semaglutide decelerates the growth and progression of breast cancer by enhancing the acquired antitumor immunity
Semaglutide, a glucagon-like peptide 1 receptor agonist, is an antidiabetic that has recently shown promising immunomodulatory and antitumor effects. Breast cancer is the most common type of cancer affecting women worldwide. The aim of this study was to analyze the effects of semaglutide on the antitumor immunity in a 4T1 mouse breast cancer model. After induction of breast cancer, BALB/C mice were treated intraperitoneally with semaglutide. Semaglutide administration decelerated tumor appearance, growth and progression. The antidiabetic drug showed neither a direct cytotoxic effect in vitro, nor an angiogenic effect. Furthermore, depletion of NK cells had no affect on tumor growth in semaglutide treated mice suggesting a non-NK cell-dependent mechanism. However, semaglutide increased the accumulation and maturation of CD11c+ dendritic cell, while decreasing the percentage of FoxP3+ regulatory T cells in the spleen and primary tumor. In addition, semaglutide increased tumor infiltration and promoted the antitumor phenotype of T cells, in vivo. Furthermore, semaglutide enhanced the cytotoxic capacity of CD8+ T cells, in vitro. These results suggest that semaglutide enhances the acquired antitumor immune response and has potential for the future treatment of malignancies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.90
自引率
2.70%
发文量
1621
审稿时长
48 days
期刊介绍:
Biomedicine & Pharmacotherapy stands as a multidisciplinary journal, presenting a spectrum of original research reports, reviews, and communications in the realms of clinical and basic medicine, as well as pharmacology. The journal spans various fields, including Cancer, Nutriceutics, Neurodegenerative, Cardiac, and Infectious Diseases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


