环黄芪醇通过促进自噬和减少 Scrib 驱动的 ROS,减少帕金森病模型中小胶质细胞 NLRP3 炎性体的激活。
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景:在帕金森病(PD)中,小胶质细胞自噬是维持细胞氧化还原平衡的关键。同时,黄芪的主要活性成分环黄芪皂苷(CAG)可降低NLRP3炎性体的活化。目的:本研究探讨了CAG在激活小胶质细胞NLRP3炎症小体中的作用及其治疗PD的潜在机制:研究设计:在α-Syn诱导的原发性小胶质细胞和PD模型中评估CAG的作用:方法:将 AAV1/2-hsyn-SNCA (A53T) 立体注射到小鼠纹状体以诱导 PD 模型,并口服 CAG。对小鼠进行定量 4D 蛋白质组学分析和行为评估。原代小胶质细胞和神经元培养物通过 Western 印迹、免疫荧光、透射电子显微镜等进行分析。结果:CAG 通过抑制小胶质细胞 Scribble (Scrib) 和 p22phox 的表达,减少了吞噬诱导的活性氧(ROS)。同时,CAG 还能增强自噬、促进 α-Syn 清除并减少线粒体损伤。这些协同作用降低了 NLRP3 炎性体的激活,进而减少了 gasdermin D 的裂解、caspase-1 的激活以及白细胞介素-1β 和白细胞介素-18 的释放。进一步研究发现,CAG能保护神经元免受α-Syn毒性的影响,从而减轻在小鼠帕金森病模型中观察到的行为障碍:结论:CAG通过促进小胶质细胞自噬和降低与Scrib相关的烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶的活性,抑制ROS诱导的小胶质细胞NLRP3炎性体的激活,从而缓解神经炎症,是治疗帕金森病的一种很有前景的替代方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
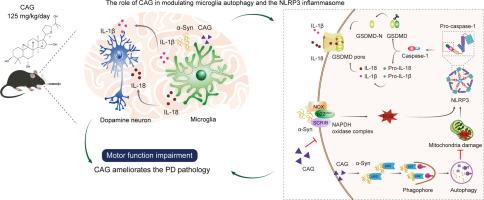
Cycloastragenol reduces microglial NLRP3 inflammasome activation in Parkinson's disease models by promoting autophagy and reducing Scrib-driven ROS
Background
In Parkinson's disease (PD), microglial autophagy is crucial for the maintenance of cellular redox homeostasis. Meanwhile, cycloastragenol (CAG), a triterpenoid saponin and the principal active component of Astragalus, reduces the activation of NLRP3 inflammasomes. Nevertheless, the specific molecular mechanisms underlying the CAG-mitigated microglial neuroinflammation remains obscure in PD.
Purpose
This study explored the role of CAG in the activation of microglial NLRP3 inflammasome and the mechanisms underlying its therapeutic potential for PD treatment.
Study design
The effect of CAG was assessed in α-Syn-induced primary microglia and PD models.
Methods
AAV1/2-hsyn-SNCA (A53T) was stereo-injected into the striatum of mice to induce PD models and CAG was orally administered. The mice underwent quantitative 4D proteomics analysis and behavioral assessments. The primary microglia and neuron cultures were analyzed by western blotting, immunofluorescence, transmission electron microscopy, etc.
Results
CAG reduced phagocytosis-induced reactive oxygen species (ROS) by suppressing the microglial Scribble (Scrib) and p22phox expression. Concurrently, CAG enhanced autophagy, promoted α-Syn clearance, and reduced mitochondrial damage. These synergistic effects downregulated NLRP3 inflammasome activation, in turn reducing gasdermin D cleavage, caspase-1 activation, and the release of interleukin-1β and interleukin-18. Further investigation revealed that CAG shielded neurons from α-Syn toxicity, thus attenuating behavioral impairments observed in the mouse PD model.
Conclusion
CAG mitigates neuroinflammation by inhibiting ROS-induced NLRP3 inflammasome activation in microglia via promoting microglial autophagy and reducing the activity of Scrib-associated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which signifies a promising alternative approach to PD management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


