磷酸戊糖途径的代谢转换会诱导胰腺癌的辐射抗性。
IF 4.9
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
目的:胰腺导管腺癌(PDAC)对包括放疗在内的标准疗法具有明显的耐药性。我们假设新陈代谢重编程可能是 PDAC 放射抗性的基础,而且有可能利用这些新陈代谢变化达到治疗目的:我们通过将 AsPC-1 和 MIAPaCa-2 人类胰腺癌细胞暴露于递增剂量的辐射中,建立了两种匹配的放射抗性 PDAC 细胞模型。我们使用 Nanostring 技术、液相色谱-质谱法标记葡萄糖追踪、海马分析法和暴露于代谢抑制剂对亲代细胞和抗放射细胞的代谢概况进行了研究。通过向裸鼠皮下注射放射抗性-AsPC-1 细胞建立的异种移植模型,评估了辐射与磷酸戊糖途径抑制剂 6-aminonicotinamide (6-AN) 的协同效应:结果:抗放射细胞过量表达丙酮酸脱氢酶激酶(PDK),并持续显示糖酵解增加,三羧酸(TCA)循环和氧化磷酸化下调。通过磷酸戊糖途径(PPP)的新陈代谢通量增加,还原型谷胱甘肽的水平也增加了;对磷酸戊糖途径的药物抑制显著增强了辐射诱导的细胞死亡。此外,辐射与 PPP 抑制剂 6-AN 的联合治疗可协同抑制体内肿瘤的生长:我们从机理上理解了导致 PDAC 产生放射抗性的代谢变化。此外,我们还证明了胰腺癌细胞可以通过代谢操作,特别是抑制 PPP,重新对辐射敏感。利用放射抗性胰腺癌细胞的代谢弱点是治疗胰腺癌的一种新方法,有望改善临床疗效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
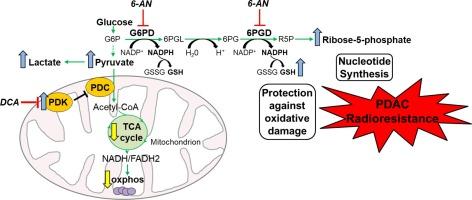
A metabolic switch to the pentose-phosphate pathway induces radiation resistance in pancreatic cancer
Purpose
Pancreatic ductal adenocarcinoma (PDAC) is remarkably resistant to standard modalities, including radiotherapy. We hypothesized that metabolic reprogramming may underlie PDAC radioresistance, and moreover, that it would be possible to exploit these metabolic changes for therapeutic intent.
Methods and materials
We established two matched models of radioresistant PDAC cells by exposing the AsPC-1 and MIAPaCa-2 human pancreatic cancer cells to incremental doses of radiation. The metabolic profile of parental and radioresistant cells was investigated using Nanostring technology, labeled-glucose tracing by liquid chromatography-mass spectrometry, Seahorse analysis and exposure to metabolic inhibitors. The synergistic effect of radiation combined with a pentose-phosphate pathway inhibitor, 6-aminonicotinamide (6-AN) was evaluated in a xenograft model established by subcutaneous injection of radioresistant-AsPC-1 cells into nude mice.
Results
The radioresistant cells overexpressed pyruvate dehydrogenase kinase (PDK) and consistently, displayed increased glycolysis and downregulated the tricarboxylic acid (TCA) cycle and oxidative phosphorylation. Metabolic flux through the pentose-phosphate pathway (PPP) was increased, as were levels of reduced glutathione; pharmacological inhibition of the PPP dramatically potentiated radiation-induced cell death. Furthermore, the combined treatment of radiation with the PPP inhibitor 6-AN synergistically inhibited tumor growth in-vivo.
Conclusions
We provide a mechanistic understanding of the metabolic changes that underlie radioresistance in PDAC. Furthermore, we demonstrate that pancreatic cancer cells can be re-sensitized to radiation via metabolic manipulation, in particular, inhibition of the PPP. Exploitation of the metabolic vulnerabilities of radioresistant pancreatic cancer cells constitutes a new approach to pancreatic cancer, with a potential to improve clinical outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Radiotherapy and Oncology
医学-核医学
CiteScore
10.30
自引率
10.50%
发文量
2445
审稿时长
45 days
期刊介绍:
Radiotherapy and Oncology publishes papers describing original research as well as review articles. It covers areas of interest relating to radiation oncology. This includes: clinical radiotherapy, combined modality treatment, translational studies, epidemiological outcomes, imaging, dosimetry, and radiation therapy planning, experimental work in radiobiology, chemobiology, hyperthermia and tumour biology, as well as data science in radiation oncology and physics aspects relevant to oncology.Papers on more general aspects of interest to the radiation oncologist including chemotherapy, surgery and immunology are also published.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


