优化原代背根神经节细胞培养方案,实现可靠的 K+ 电流膜片钳记录。
IF 2.5
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
源自啮齿动物的 DRG 原始神经元培养物与体内感觉神经元的特性非常相似,对研究疼痛和神经系统疾病非常有用。这些培养物在用于感觉神经元特性分析的膜片钳电生理学中至关重要。科学研究中详细、可复制的实验方案可确保实验的准确性和可重复性。本文详细介绍了如何复制用于膜片钳记录的原代 DRG 细胞培养方案并获得一致的结果。我们概述了建立原代 DRG 细胞培养的综合方案,该方案经过优化,可改善全细胞膜片钳记录中的千粒重形成。此外,我们还进行了一项模拟研究,重点是记录宏观 K+ 通道。研究结果确定了一个优化的新方案,该方案可可靠地使用本出版物中描述的原代 DRG 细胞进行全细胞膜片钳记录和数据分析。文献中有关该方案的细节分散在不同的出版物中,因此很难在一个来源中找到全面的总结。与文献中的现有方法相比,本研究首次证实了使用较少的方案步骤的有效性,这减少了获得合适的膜片钳记录细胞的压力和变异性。鉴于原代 DRG 细胞解离过程所带来的挑战,以及文献中全面方法记录的重要性,本研究提出的方案改进了原代 DRG 细胞培养在膜片钳记录中的应用,并使之保持一致。本文章由计算机程序翻译,如有差异,请以英文原文为准。
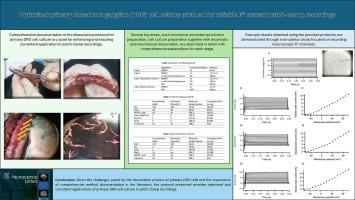
Optimized primary dorsal root ganglion cell culture protocol for reliable K+ current patch-clamp recordings
DRG primary neuron cultures, derived from rodents, closely mimic properties of sensory neurons in vivo and are highly useful for studying pain and neurological disorders. These cultures are pivotal in patch-clamp electrophysiology for sensory neuron properties analysis. A detailed, replicable protocol in scientific research ensures experiment accuracy and reproducibility. This paper provides comprehensive details for replicating the protocol and achieving consistent results in primary DRG cell culture as used for patch-clamp recordings. We outlined a comprehensive protocol for establishing primary DRG cell culture, optimized for improved gigaseal formation in whole-cell patch-clamp recordings. Additionally, we conducted a simulation study focused on recording macroscopic K+ channels. The findings established an optimized novel protocol that works reliably for whole-cell patch-clamp recordings and data analysis using primary DRG cells prepared as described in this publication. The details for the protocol in the literature are dispersed across various publications, making it challenging to find a comprehensive summary in one source. This study confirms, for the first time, the efficacy of using fewer protocol steps, which reduces stress and variability in obtaining suitable cells for patch-clamp recordings compared to existing methods in the literature. Given the challenges posed by the dissociation process of primary DRG cells and the importance of comprehensive method documentation in the literature, the protocol presented provides improved and consistent applications of primary DRG cell culture in patch-clamp recordings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuroscience Letters
医学-神经科学
CiteScore
5.20
自引率
0.00%
发文量
408
审稿时长
50 days
期刊介绍:
Neuroscience Letters is devoted to the rapid publication of short, high-quality papers of interest to the broad community of neuroscientists. Only papers which will make a significant addition to the literature in the field will be published. Papers in all areas of neuroscience - molecular, cellular, developmental, systems, behavioral and cognitive, as well as computational - will be considered for publication. Submission of laboratory investigations that shed light on disease mechanisms is encouraged. Special Issues, edited by Guest Editors to cover new and rapidly-moving areas, will include invited mini-reviews. Occasional mini-reviews in especially timely areas will be considered for publication, without invitation, outside of Special Issues; these un-solicited mini-reviews can be submitted without invitation but must be of very high quality. Clinical studies will also be published if they provide new information about organization or actions of the nervous system, or provide new insights into the neurobiology of disease. NSL does not publish case reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


