通过 DES 的形成提高胡椒碱的溶解度:通过计算和光谱方法阐明分子间相互作用以及对应结构的影响。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
开发理化性质更强的现有原料药新形式对于提高其治疗潜力至关重要。在此背景下,离子液体(IL)和深共晶溶剂(DES)因其独特的性质和提高溶解度的潜力,近年来受到了广泛关注。在本研究中,我们探讨了不同对映体在与哌啶(PI)(一种水溶性较差的药物)形成 IL/DESs 过程中的作用。在筛选了 14 种对应分子后,我们开发并研究了 10 种液态 PI 对应体系。热分析证实了 IL/DES 的形成,而计算和光谱研究则揭示了氢键在 PI 与对应分子之间的相互作用中起着关键作用,从而证实了 DES 的形成。PI 在这些体系中的溶解度提高了 36% 至 294%,其中 PI-邻苯二甲酸(OA)的饱和溶解度最高(49.71 μg/mL),PI-布洛芬(IB)的饱和溶解度最低(17.23 μg/mL)。对等物中氢键基团的存在是成功形成 DES 的关键。溶解度与 logP 之间呈负相关(r = - 0.75,p* = 0.0129),而溶解度与归一化极性表面积 (PSA) 之间呈正相关(r = 0.68,p* = 0.029)。PI-OA 和 PI-IB 位于这些回归线的两端,进一步验证了这些特性与溶解度增强之间的关系。这些发现凸显了合理的 IL/DES 设计的重要方面,优化了它们的性能,使其应用更加广泛。本文章由计算机程序翻译,如有差异,请以英文原文为准。
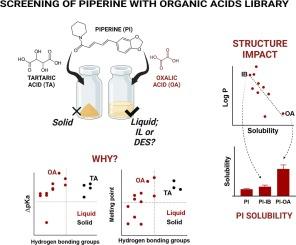
Piperine solubility enhancement via DES formation: Elucidation of intermolecular interactions and impact of counterpart structure via computational and spectroscopic approaches
The development of new forms of existing APIs with enhanced physicochemical properties is critical for improving their therapeutic potential. In this context, ionic liquids (ILs) and deep eutectic solvents (DESs) have gained significant attention in recent years due to their unique properties and potential for solubility enhancement. In this study, we explore the role of different counterparts in the formation of IL/DESs with piperine (PI), a poorly water-soluble drug. After screening a library of fourteen counterpart molecules, ten liquid PI-counterpart systems were developed and investigated. Thermal analysis confirmed the formation of IL/DES, while computational and spectroscopic studies revealed that hydrogen bonding played a crucial role in the interaction between PI and the counterparts, confirming DES formation. The solubility enhancement of PI in these systems ranged from ∼ 36 % to 294 %, with PI-Oxalic acid (OA) exhibiting the highest saturation solubility (49.71 μg/mL) and PI-Ibuprofen (IB) the lowest (17.23 μg/mL). The presence of hydrogen bonding groups in counterparts was key to successful DES formation. A negative correlation was observed between solubility and logP (r = − 0.75, p* = 0.0129), while a positive correlation was found between solubility and normalized polar surface area (PSA) (r = 0.68, p* = 0.029). PI-OA and PI-IB were located at the extreme ends of these regression lines, further validating the relationship between these properties and solubility enhancement. These findings highlight essential aspects of rational IL/DES design, optimizing their properties for broader applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


