纤维素纳米晶/纤维素纳米纤丝-组合虾青素纳米乳液用于加强胃癌细胞的肿瘤靶向递送。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
本研究采用基于纳米乳液的纳米材料(NE)来评估其对胃癌细胞的疗效和抗肿瘤潜力。用虾青素/α-生育酚-纤维素纳米晶/纤维素纳米纤丝制备了用于治疗胃癌的纳米乳液。采用标准方法测试了其对癌细胞的细胞毒性潜力,并从细胞增殖、迁移和细胞摄取等方面进行了评估。在异种移植小鼠模型中检验了 NE 与光动力疗法(PDT)的协同作用。结果证实了光动力疗法和 NEs 在体内动物模型中的协同作用。受调控的蛋白质表达表现出毒性降低以及对细胞增殖和迁移的抑制作用。抗肿瘤研究表明,NE 可抑制体内人结肠癌的生长。免疫组织学分析证实了 NE 对 PI3K/AKT 信号通路的调节作用。本研究表明,NE 可通过免疫调节信号通路增强对人类胃癌的抗癌效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
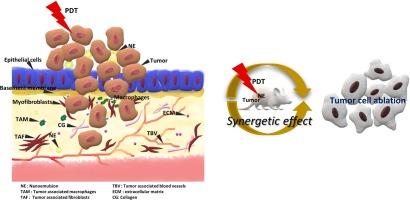
Cellulose nanocrystals/cellulose nanofibrils-combined astaxanthin nanoemulsion for reinforcement of targeted tumor delivery of gastric cancer cells
Nanoemulsion based nanomaterial (NE) was carried out in the present study to evaluate the efficacy and its antitumor potential of the gastric cancer cells. NE was prepared with astaxanthin/alpha-tocopherol- cellulose nanocrystals/cellulose nanofibrils based nanoemulsions for gastric cancer treatment. The cytotoxic potential was tested against cancer cells and evaluated in terms of its cell proliferation, migration, and cellular uptake by the standard methods. NE was examined for its synergetic effect with photodynamic therapy (PDT) in a xenograft mouse model. The results confirmed the synergetic effect of PDT and NEs in the in vivo animal model. The regulated expression of proteins manifested the reduced toxicity and inhibition of cell proliferation and migration. The antitumor study showed that NE inhibited the growth of human colon cancer in vivo. Immunohistological analysis confirmed the regulation of PI3K/AKT signaling pathway. The present study demonstrates that NEs can enhance anti-cancer effect against human gastric cancer through the immunomodulatory signaling pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


