热熔挤压法制备的两性聚合物辅料和吲哚美辛无定形固体分散体。
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
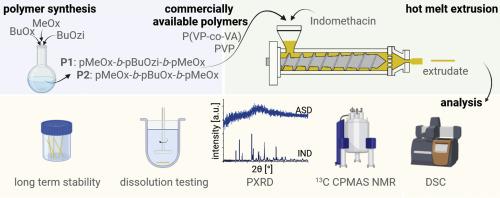
提高水溶性差的药物的溶解度对于提高生物利用度、配方灵活性和减少患者之间的差异至关重要。制备无定形固体分散体(ASD)是配制此类药物的一种有吸引力的策略,可提高表观水溶性,从而提高生物利用率。对于此类 ASD,水溶性聚合物辅料,如聚(乙烯基吡咯烷酮)(PVP)或聚(乙烯基吡咯烷酮-醋酸乙烯酯)(P(VP-co-VA))可用于溶解和稳定药物,防止结晶。我们认为,含有叔酰胺的聚合物特别适合稳定含有 H 键供体的药物,因为它们在聚合物和药物之间提供了强大的 H 键潜力。本研究的目的是比较作为 ASD 赋形剂的新型和成熟的叔酰胺聚合物。实验性两亲 ABA 三嵌段共聚物由聚(2-甲基-2-恶唑啉)(pMeOx)、聚(2-丁基-2-恶唑啉)(pBuOx)和聚(2-丁基-2-恶嗪)(pBuOzi)嵌段组成,并与已有的赋形剂 PVP 和 P(VP-co-VA)进行了比较。以吲哚美辛为模型药物,通过热熔挤出法制备了高药物载量的 ASD。使用 DSC 和 PXRD 对挤出物进行了研究,结果表明,在吲哚美辛含量达到 75wt% 时,ASD 完全无定形,与所使用的聚合物无关。13C CPMAS NMR 深入揭示了分子间关联与药物负载量的关系,并表明在 pMeOx-pBuOzi-pMeOx 和 pMeOx-pBuOx-pMeOx 中,当药物负载量为 75wt% 时存在药物二聚体,这可能会影响物理稳定性。与聚合物无关,ASD 中药物的固态形式也会影响样品的溶解曲线,因为含有结晶吲哚美辛的样品比完全无定形的样品溶解速度更慢。这项研究表明,聚(2-噁唑啉)和聚(2-噁嗪)聚合物与 PVP 和 P(VP-co-VA)相似,是制备 ASD 的有效聚合物,值得进一步研究这些新型聚合物用于配制 ASD。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Amorphous solid dispersions of amphiphilic polymer excipients and indomethacin prepared by hot melt extrusion
Improving the solubility of poorly water-soluble drugs is essential for enhancing bioavailability, formulation flexibility and reducing patient-to-patient variability. The preparation of amorphous solid dispersions (ASDs) is an attractive strategy to formulate such drugs, leading to higher apparent water solubility and therefore higher bioavailability. For such ASDs, water-soluble polymer excipients, such as poly(vinyl pyrrolidone) (PVP) or poly(vinyl pyrrolidone-co-vinyl acetate) (P(VP-co-VA)), are employed to solubilize and stabilize the drug against crystallization. We posit that polymers bearing tertiary amides are particularly well suited to stabilizing drugs containing H-bond donors, as they offer strong H-bonding potential between the polymer and drug. The aim of this study was to compare new and established polymers with tertiary amides as excipients for ASDs. Experimental amphiphilic ABA triblock copolymers comprising poly(2-methyl-2-oxazoline) (pMeOx), poly(2‑butyl‑2-oxazoline) (pBuOx) and poly(2‑butyl‑2-oxazine) (pBuOzi) blocks, were compared with the established excipients, PVP and P(VP-co-VA). ASDs with indomethacin as the model drug were prepared at high drug loadings via hot melt extrusion. The extrudates were studied with DSC and PXRD, revealing the ASDs to be fully amorphous up to 75wt% indomethacin, independent of the polymer used. 13C CPMAS NMR provided insights into intermolecular associations as a function of drug loading, and suggested the presence of drug dimers at 75wt% drug loading in pMeOx-pBuOzi-pMeOx and pMeOx-pBuOx-pMeOx, which could affect physical stability. Independent of the polymers, the solid-state form of the drug in the ASD was found to affect the dissolution profile of the samples, insofar as the samples containing crystalline indomethacin showed slower dissolution than the fully amorphous ones. This study shows that the polymers comprising poly(2-oxazoline) and poly(2-oxazine) are effective polymers for ASD preparation, similar to PVP and P(VP-co-VA) which merits further investigations into these novel polymers for formulating ASDs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


