单细胞转录组学揭示了人类 iPS 细胞分化成外胚层眼系的分子基础。
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
由人类诱导多能干细胞(hiPSCs)生成的自形成、外胚层、自主多区(SEAM)为研究眼部细胞随时间分化的动态提供了一个独特的视角。在这里,通过利用单细胞转录组学,我们(i)识别了(ii)分子特征,(iii)确定了在SEAM形成过程中出现的外胚层衍生眼细胞群的发育轨迹。我们的分析揭示了早期眼组织之间的相互依存关系,并勾勒出了12周内不同细胞类型的顺序形成和成熟过程。我们展示了从多能性到组织规格化和分化的过程,其中包括表面外胚层和神经外胚层眼系,以及发育中角膜、结膜、晶状体和视网膜的 iPSC 派生成分的生成。我们的研究结果不仅促进了对基于干细胞的人源系统中眼部发育的理解,还建立了一个强大的方法范例,用于在单细胞分辨率下探索SEAM形成过程中的细胞和分子动态,并强调了hiPSC衍生系统作为模拟人眼发育和疾病的强大平台的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
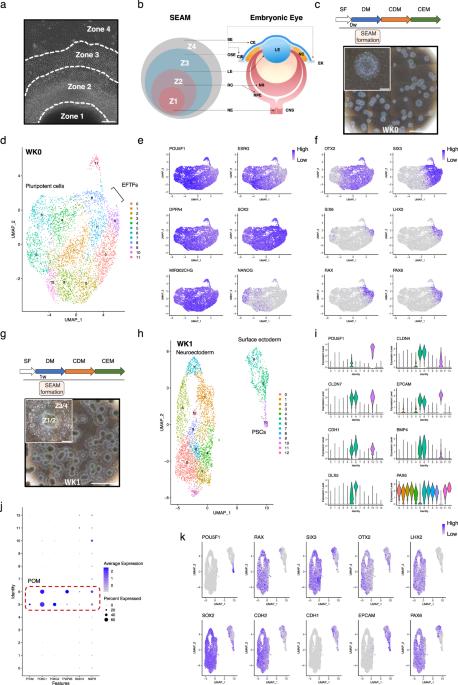
Single-cell transcriptomics reveals the molecular basis of human iPS cell differentiation into ectodermal ocular lineages
The generation of a self-formed, ectodermal, autonomous multi-zone (SEAM) from human induced pluripotent stem cells (hiPSCs) offers a unique perspective to study the dynamics of ocular cell differentiation over time. Here, by utilising single-cell transcriptomics, we have (i) identified, (ii) molecularly characterised and (iii) ascertained the developmental trajectories of ectodermally-derived ocular cell populations which emerge within SEAMs as they form. Our analysis reveals interdependency between tissues of the early eye and delineates the sequential formation and maturation of distinct cell types over a 12-week period. We demonstrate a progression from pluripotency through to tissue specification and differentiation which encompasses both surface ectodermal and neuroectodermal ocular lineages and the generation of iPSC-derived components of the developing cornea, conjunctiva, lens, and retina. Our findings not only advance the understanding of ocular development in a stem cell-based system of human origin, but also establish a robust methodological paradigm for exploring cellular and molecular dynamics during SEAM formation at single-cell resolution and highlight the potential of hiPSC-derived systems as powerful platforms for modelling human eye development and disease. Single-cell transcriptomic analyses delineate the differentiation of human iPSCs into ectodermal ocular lineages, highlighting defined developmental trajectories and the sequential formation and maturation of distinct ocular cell types in vitro.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


