综合多组学确定了烟曲霉和肺炎双球菌种间相互作用的途径。
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
涉及细菌和真菌病原体的多菌共感染和超级感染给诊断和治疗带来了严峻的挑战,并与发病率和死亡率升高有关。然而,细菌与真菌相互作用(BFI)的代谢动态及其对疾病结果的影响在很大程度上仍不为人所知。真菌烟曲霉和肺炎克雷伯氏菌是临床上重要的病原体,它们在人体内,尤其是下呼吸道中有着共同的生态位。我们利用综合多组学方法揭示了这些病原体在混合生物膜中进行种间交流的复杂而多面的过程。在这种情况下,烟曲霉通过重构新陈代谢、削弱翻译机制以及连接次级和初级新陈代谢来应对细菌的挑战,而肺炎双球菌则保持其中心新陈代谢和翻译活动。烟曲霉新陈代谢的灵活性以及快速适应由细菌介导的不断变化的微环境的能力,为研究相互竞争的相互作用伙伴之间的交叉交流的影响提供了新的可能性。这些数据强调了 BFI 动态变化的复杂性,例如烟曲霉与肺炎双球菌相互作用时发生的明显代谢变化。我们的研究结果确定了候选生物标记物,这些标记物有可能用于改善 BFI 的临床管理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
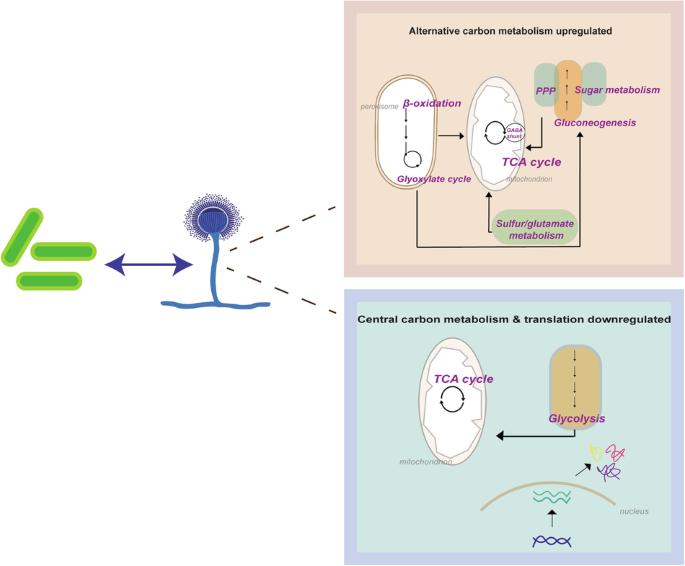
Integrated multi-omics identifies pathways governing interspecies interaction between A. fumigatus and K. pneumoniae
Polymicrobial co- and superinfections involving bacterial and fungal pathogens pose serious challenges for diagnosis and therapy, and are associated with elevated morbidity and mortality. However, the metabolic dynamics of bacterial–fungal interactions (BFI) and the resulting impact on disease outcome remain largely unknown. The fungus Aspergillus fumigatus and the bacterium Klebsiella pneumoniae are clinically important pathogens sharing common niches in the human body, especially in the lower respiratory tract. We have exploited an integrated multi-omics approach to unravel the complex and multifaceted processes implicated in the interspecies communication involving these pathogens in mixed biofilms. In this setting, A. fumigatus responds to the bacterial challenge by rewiring its metabolism, attenuating the translational machineries, and by connecting secondary with primary metabolism, while K. pneumoniae maintains its central metabolism and translation activity. The flexibility in the metabolism of A. fumigatus and the ability to quickly adapt to the changing microenvironment mediated by the bacteria highlight new possibilities for studying the impact of cross-communication between competing interaction partners. The data underscore the complexity governing the dynamics underlying BFI, such as pronounced metabolic changes mounted in A. fumigatus interacting with K. pneumoniae. Our findings identify candidate biomarkers potentially exploitable for improved clinical management of BFI. A multi-omics study highlights the flexibility in the metabolism of Aspergillus fumigatus in microenvironments co-shared with Klebsiella pneumoniae, uncovering potential biomarkers to enhance clinical care for polymicrobial infections.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


