人类鼻病毒的低温电子显微镜揭示了囊膜-RNA 双链相互作用,为了解病毒组装和基因组解衣提供了线索。
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
本研究确定的人类鼻病毒 B14 的低温电子显微镜结构显示,13-bp RNA 双链对称地结合在二十面体病毒帽的 30 个二倍轴周围的每个区域。这些 RNA 双链体(约占 ssRNA 基因组的 12%)形成了一个准十二面体笼子,占据了病毒外壳内表面的很大一部分。RNA 双链体与噬菌体内壁的口袋建立了复杂的非共价相互作用网络,包括与围绕每个 RNA 双链体的一组碱性氨基酸残基的库仑相互作用。我们直接比较了充满 RNA 的病毒的冷冻电子显微镜结构和从一小部分病毒的基因组中释放出来的无 RNA(空)噬菌体的冷冻电子显微镜结构。比较结果表明,当 RNA 从噬菌体中释放出来时,病毒体中参与噬菌体-双链 RNA 相互作用的一些特定残基会发生显著的构象重排。RNA 的释放还与 30 个双折轴通道的异步打开有关。这些研究结果进一步揭示了鼻病毒粒子在感染过程中的组装及其基因组脱壳的分子机制。这些结果还可能有助于开发新的抗病毒策略,以干扰病毒感染周期中病毒外壳与基因组之间的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
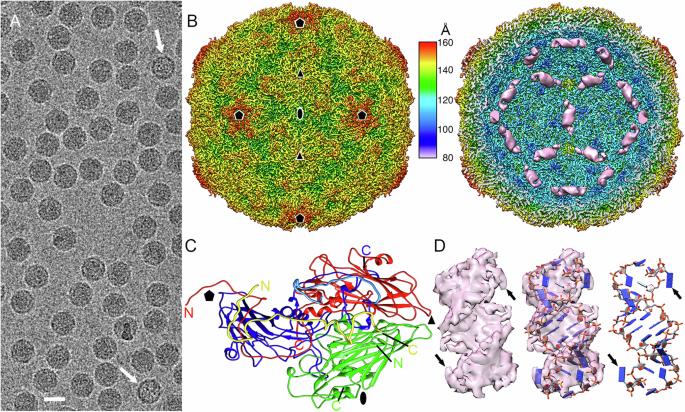
Cryo-EM of human rhinovirus reveals capsid-RNA duplex interactions that provide insights into virus assembly and genome uncoating
The cryo-EM structure of the human rhinovirus B14 determined in this study reveals 13-bp RNA duplexes symmetrically bound to regions around each of the 30 two-fold axes in the icosahedral viral capsid. The RNA duplexes (~12% of the ssRNA genome) define a quasi-dodecahedral cage that line a substantial part of the capsid interior surface. The RNA duplexes establish a complex network of non-covalent interactions with pockets in the capsid inner wall, including coulombic interactions with a cluster of basic amino acid residues that surround each RNA duplex. A direct comparison was made between the cryo-EM structure of RNA-filled virions and that of RNA-free (empty) capsids that resulted from genome release from a small fraction of viruses. The comparison reveals that some specific residues involved in capsid-duplex RNA interactions in the virion undergo remarkable conformational rearrangements upon RNA release from the capsid. RNA release is also associated with the asynchronous opening of channels at the 30 two-fold axes. The results provide further insights into the molecular mechanisms leading to assembly of rhinovirus particles and their genome uncoating during infection. They may also contribute to development of novel antiviral strategies aimed at interfering with viral capsid-genome interactions during the infectious cycle. Cryo-EM structure of human rhinovirus B-14 reveals genomic RNA duplexes that delineate a dodecahedral cage associated with the capsid inner surface, and an asynchronous opening of capsid channels that facilitate the release of the genome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


