耐青蒿素的恶性疟原虫 Kelch13 突变体蛋白显示出血红素结合亲和力降低和青蒿素激活能力下降。
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
一线抗疟药物青蒿素(ART)衍生物的药效是由血红素诱导的内过氧键裂解形成活性血红素-ART 烷氧基自由基和共价血红素-ART 加合物引发的,后者对寄生虫有剧毒。恶性疟原虫含 Kelch 蛋白 Kelch13(PfKekch13)发生突变的抗逆转录病毒寄生虫(ART-R)表现出血红蛋白摄取受损、血红蛋白衍生血红素产量减少,从而降低了 ART 的活化。然而,PfKelch13 直接参与血红素介导的 ART 激活的情况尚未见报道。在这里,我们发现纯化的重组 PfKelch13 野生型(WT)蛋白与铁和血红素(ART 激活的主要效应物)具有可测量的结合亲和力。寄生虫培养物中的原生 PfKelch13 蛋白也具有血红素结合特性。与 ART 敏感的 WT 和 A578S 突变蛋白相比,两个 ART-R 重组 PfKelch13 突变体(C580Y 和 R539T)显示出较弱的血红素结合亲和力,当 ART 与结合到突变 PfKelch13 蛋白上的血红素分子孵育时,这进一步转化为血红素-ART 衍生物产量的减少。总之,本研究首次提供了通过 PfKelch13 的血红素结合倾向激活 ART 的证据。这一机制可能有助于通过 PfKelch13 的新功能调节疟原虫体内的 ART-R 水平。本文章由计算机程序翻译,如有差异,请以英文原文为准。
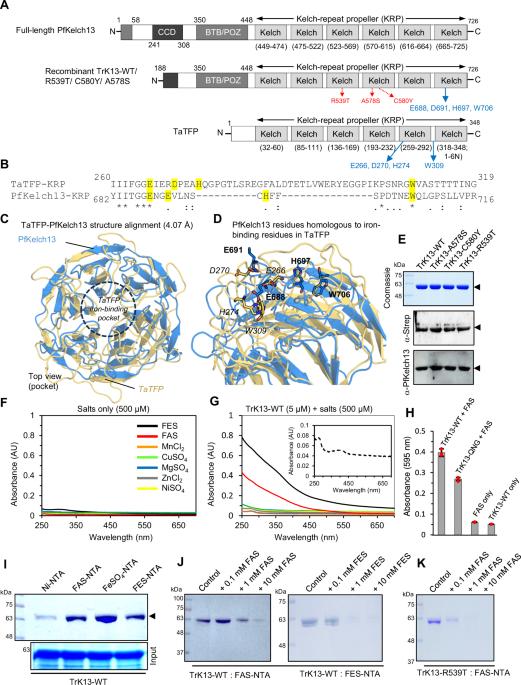
Artemisinin-resistant Plasmodium falciparum Kelch13 mutant proteins display reduced heme-binding affinity and decreased artemisinin activation
The potency of frontline antimalarial drug artemisinin (ART) derivatives is triggered by heme-induced cleavage of the endoperoxide bond to form reactive heme-ART alkoxy radicals and covalent heme-ART adducts, which are highly toxic to the parasite. ART-resistant (ART-R) parasites with mutations in the Plasmodium falciparum Kelch-containing protein Kelch13 (PfKekch13) exhibit impaired hemoglobin uptake, reduced yield of hemoglobin-derived heme, and thus decreased ART activation. However, any direct involvement of PfKelch13 in heme-mediated ART activation has not been reported. Here, we show that the purified recombinant PfKelch13 wild-type (WT) protein displays measurable binding affinity for iron and heme, the main effectors for ART activation. The heme-binding property is also exhibited by the native PfKelch13 protein from parasite culture. The two ART-R recombinant PfKelch13 mutants (C580Y and R539T) display weaker heme binding affinities compared to the ART-sensitive WT and A578S mutant proteins, which further translates into reduced yield of heme-ART derivatives when ART is incubated with the heme molecules bound to the mutant PfKelch13 proteins. In conclusion, this study provides the first evidence for ART activation via the heme-binding propensity of PfKelch13. This mechanism may contribute to the modulation of ART-R levels in malaria parasites through a novel function of PfKelch13. Elucidation of heme binding affinity and artemisinin activation by the Plasmodium falciparum Kelch13 protein variants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


