IL-1β 介导的成纤维细胞-中性粒细胞串联促进了系统性红斑狼疮皮肤病变的炎症环境。
IF 4.5
3区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
系统性红斑狼疮(SLE)的特点是免疫失调,皮肤病变中的中性粒细胞浸润导致炎症和疾病进展。然而,成纤维细胞与中性粒细胞在系统性红斑狼疮皮损中的相互作用尚未得到充分探讨。通过单细胞 RNA 测序,我们在系统性红斑狼疮皮损中发现了一个独特的 CXCL1+ 成纤维细胞亚群。我们发现,CXCL1+成纤维细胞可招募和激活中性粒细胞,增加炎症介质、活性氧和中性粒细胞胞外捕获物的产生。这些成纤维细胞还促进了中性粒细胞向低密度表型的转变。值得注意的是,这些成纤维细胞延迟了中性粒细胞的凋亡,延长了它们的存活时间并扩大了炎症。CXCL1+ 成纤维细胞分泌的血清淀粉样蛋白 A1 成为中性粒细胞的关键激活剂。活化的中性粒细胞反过来分泌 IL-1β,通过激活 NF-κB 通路诱导 CXCL1+ 成纤维细胞分化。总之,我们的研究结果表明,IL-1β诱导的CXCL1+成纤维细胞能显著调节促炎性中性粒细胞,这突显了成纤维细胞和中性粒细胞之间在系统性红斑狼疮发病机制中的关键串联作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
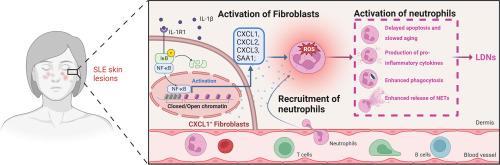
IL-1β mediated fibroblast-neutrophil crosstalk promotes inflammatory environment in skin lesions of SLE
Systemic lupus erythematosus (SLE) is characterized by immune dysregulation, with neutrophil infiltration in skin lesions contributing to inflammation and disease progression. However, the interaction between fibroblasts and neutrophils in SLE skin lesions has not been fully explored. Using single-cell RNA sequencing, we identified a unique CXCL1+ fibroblast subset in SLE lesions. We found that CXCL1+ fibroblasts recruit and activate neutrophils, increasing the production of inflammatory mediators, reactive oxygen species, and neutrophil extracellular traps. These fibroblasts also facilitated the transition of neutrophils to a low-density phenotype. Notably, these fibroblasts delayed neutrophil apoptosis, extending their survival and amplifying inflammation. Serum amyloid A1, secreted by CXCL1+ fibroblasts, emerged as a key activator of neutrophils. Activated neutrophils, in turn, secreted IL-1β to induce CXCL1+ fibroblasts differentiation via activating the NF-κB pathway. In conclusion, our findings reveal that IL-1β-induced CXCL1+ fibroblasts significantly modulate pro-inflammatory neutrophils, underscoring the critical crosstalk between fibroblasts and neutrophils in SLE pathogenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Clinical immunology
医学-免疫学
CiteScore
12.30
自引率
1.20%
发文量
212
审稿时长
34 days
期刊介绍:
Clinical Immunology publishes original research delving into the molecular and cellular foundations of immunological diseases. Additionally, the journal includes reviews covering timely subjects in basic immunology, along with case reports and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


