饮用水中 2,2,4-三溴-5-羟基环戊-4-烯-1,3-二酮的结构确认、反应性、细菌诱变性和定量。
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
最近,通过非目标分析,英国发现了两种新的消毒剂副产物(DBP),即卤代羟基环戊二烯和卤代甲磺酸。在这项工作中,我们确认了 2,2,4-三溴-5-羟基环戊-4-烯-1,3-二酮(TBHCD)的结构,并对其与二溴甲基磺酸进行了定量,在伦敦饮用水中的平均含量分别为 122 ± 34 和 326 ± 157 纳克/升(n = 21)。我们发现,TBHCD 在自来水和碱性 pH 值条件下具有光不稳定性。此外,TBHCD 和其他三种卤代羟基环戊二烯的光谱和计算数据表明,它们可以成为水中和体内的自由基来源。重要的是,经计算,TBHCD 的 C-Br 键解离焓比有机合成中常用的自由基取代试剂 N-bromosuccinimide 的 N-Br 键低 14.5 kcal mol-1。在使用 TA98、TA100 和 TA102 菌株进行的沙门氏菌/微粒体试验中,TBHCD 具有致突变性。这项工作揭示了三卤代羟基环戊-4-烯-1,3-二酮的独特特征、活性和毒性,促使人们需要更全面地评估其风险。本文章由计算机程序翻译,如有差异,请以英文原文为准。
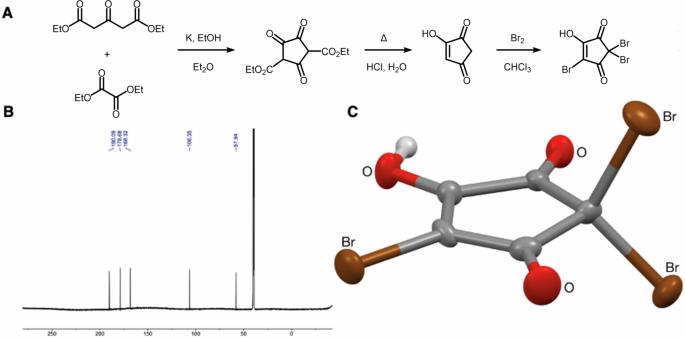
Structure confirmation, reactivity, bacterial mutagenicity and quantification of 2,2,4-tribromo-5-hydroxycyclopent-4-ene-1,3-dione in drinking water
The presence of two new disinfectant by-product (DBP) groups in the UK was recently shown using non-target analysis, halogenated-hydroxycyclopentenediones and halogenated-methanesulfonic acids. In this work, we confirmed the structure of 2,2,4-tribromo-5-hydroxycyclopent-4-ene-1,3-dione (TBHCD), and quantified it together with dibromomethanesulfonic acid at 122 ± 34 and 326 ± 157 ng L−1 on average in London’s drinking water, respectively (n = 21). We found TBHCD to be photolabile and unstable in tap water and at alkaline pH. Furthermore, spectral and computational data for TBHCD and three other halogenated-hydroxycyclopentenediones indicated they could act as a source of radicals in water and in the body. Importantly, TBHCD was calculated to have a 14.5 kcal mol−1 lower C-Br bond dissociation enthalpy than the N-Br bond of N-bromosuccinimide, a common radical substitution reagent used in organic synthesis. TBHCD was mutagenic in Salmonella/microsome assays using strains TA98, TA100 and TA102. This work reveals the unique features, activity and toxicity of trihalogenated hydroxycyclopent-4-ene-1,3-diones, prompting a need to more comprehensively assess their risks. Halogenated disinfection by-products are a recognized health risk, but unequivocal identification and monitoring of new compounds is challenging, which prevents risk assessment. Here, the authors identify and quantify 2,2,4-tribromo-5-hydroxycyclopent-4-ene-1,3-dione in London drinking water, and describe the compound’s activity and toxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


