伴侣介导的自噬(CMA)赋予 HBO 预处理对中风的神经保护作用。
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
以往通过研究缺血级联和设计抑制这些通路的方法来确定急性缺血性中风的神经保护靶点的尝试在转化研究中失败了。我们假设,通过一个成熟的范例研究内源性神经保护的分子决定因素,即高压氧(HBO)预处理对缺血性中风的耐受性,将发现新的神经保护靶点。通过结合蛋白质组学、KEGG通路分析、溶酶体组分和Western印迹分析,我们发现HBO预处理激活了伴侣介导的自噬(CMA)。此外,LAMP2A 在中风早期的皮层神经元中独特地减少。用重组腺相关病毒载体(rAAV)介导的针对 LAMP2A 转录本的短发夹核糖核酸(shRNAs)抑制 CMA 会增加神经元凋亡的易感性,并取消 HBO 预处理诱导的神经保护作用。临床上使用的 FDA 批准药物 mycophenolate mofetil 能以 CMA 依赖性方式诱导脑卒中后的长期神经保护。总之,HBO预处理通过诱导CMA获得针对缺血的神经保护作用,是一种很有前景的脑卒中转化治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
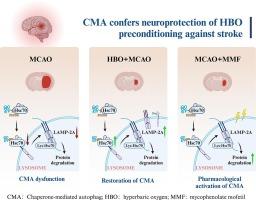
Chaperone-mediated autophagy (CMA) confers neuroprotection of HBO preconditioning against stroke
Previous attempts to identify neuroprotective targets for acute ischemic stroke by studying ischemic cascades and devising ways to suppress these pathways have failed in translational research. We hypothesized that studying the molecular determinants of endogenous neuroprotection, namely, the tolerance against ischemic stroke conferred by hyperbaric oxygen (HBO) preconditioning, via a well-established paradigm would reveal new neuroprotective targets. By a combination of proteomics, KEGG pathway analysis, lysosome fraction and western blot analysis, we found that chaperone-mediated autophagy (CMA) was activated by HBO preconditioning. In addition, LAMP2A is uniquely decreased in cortical neurons in the early stage of stroke. Suppression of CMA with recombinant adeno-associated viral vector (rAAV)-mediated delivery of short hairpin RNAs (shRNAs) targeting the LAMP2A transcript increased the neuronal susceptibility of apoptosis and abolished the neuroprotection induced by HBO preconditioning. Administration of the clinically utilized FDA-approved drug mycophenolate mofetil induced long-term neuroprotection post-stroke in a CMA-dependent manner. In summary, HBO preconditioning confers neuroprotection against ischemia by inducing CMA, which is a promising translational treatment target for stroke.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


