基于生物仿生微粒的 UC 间充质干细胞通过其优势特性对急性脑部炎症产生了良好的调节作用。
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
活化的小胶质细胞引发的神经炎症会导致神经元损伤,并在一定程度上导致神经变性。人脐带间充质干细胞(UC-MSCs)具有良好的免疫调节和神经保护作用,并具有治疗神经炎症相关疾病的潜力。然而,神经炎症造成的复杂微环境对移植的 UC-MSCs 构成了挑战。新兴的基于仿生微米(BN)的培养技术为优化 UC 间充质干细胞的制备提供了新的机会;但基于 BN 的 UC 间充质干细胞特性的根本变化仍不清楚,需要更可靠的临床前数据来支持其调节炎症的能力。在这里,我们系统研究了传统静态平面培养(SP-UCMSCs)和基于 BN 的悬浮培养(BN-UCMSCs)中 UC-MSCs 的细胞特性和炎症调节能力。在体外,与 SP-UCMSCs 相比,BN-UCMSCs 不仅保持了间充质干细胞的基本特性,还显著增强了细胞增殖、粘附和迁移等能力;特别是,BN-UCMSCs 的旁分泌功能和抗炎能力也得到了增强。我们进一步建立了小鼠急性脑部炎症模型,结果表明,与 SP-UCMSCs 组相比,BN-UCMSCs 组海马和皮质组织中促炎细胞因子的表达水平明显降低。随后对海马和大脑皮层组织进行的转录组学分析表明,BN-UCMSCs 具有通过 TLR4-Myd88-NF-κB 轴显著减少促炎细胞因子表达的优势,这一点在基因和蛋白质水平上得到了进一步验证。总之,这些数据有力地表明,BN-UCMSCs 通过其优势特性对急性脑部炎症产生了良好的调节作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
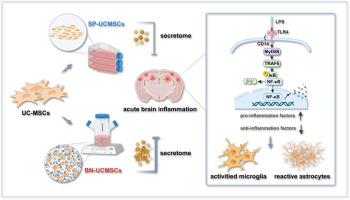
UC-MSCs based on biomimetic microniche exert excellent regulatory effects on acute brain inflammation through advantageous properties
Neuroinflammation triggered by activated microglia leads to neuronal damage and, to a certain extent, neurodegeneration. Human umbilical cord mesenchymal stem cells (UC-MSCs) have good immunomodulatory and neuroprotective effects as well as therapeutic potential for neuroinflammation-related diseases. However, the complex microenvironment created by neuroinflammation poses a challenge to transplanted UC-MSCs. The emerging biomimetic microniche (BN)-based culture technology provides new opportunities to optimize the preparation of UC-MSCs; but the fundamental changes in the characteristics of UC-MSCs based on BN remain unclear, and more reliable preclinical data are needed to support their ability to regulate inflammation. Here, we systematically studied the cellular properties and inflammation regulatory capacity of UC-MSCs in conventional static planar culture (SP-UCMSCs) and suspension culture based on BN (BN-UCMSCs). In vitro, compared with SP-UCMSCs, BN-UCMSCs not only maintained the fundamental characteristics of MSCs, but also significantly enhanced cell proliferation, adhesion, and migration capabilities, etc; notably, the paracrine function and anti-inflammatory capacity of BN-UCMSCs were also enhanced. We further established a murine model of acute brain inflammation and demonstrated that the expression level of pro-inflammatory cytokines in hippocampal and cortical tissues of the BN-UCMSCs group was significantly decreased compared with that in the SP-UCMSCs group. Subsequent transcriptomic analysis of hippocampal and cortical tissues revealed that BN-UCMSCs had the advantage of significantly reducing the expression of pro-inflammatory cytokines through the TLR4-Myd88-NF-κB axis, which was further validated at the gene and protein levels. Taken together, these data strongly indicated that BN-UCMSCs exerts excellent regulatory effects on acute brain inflammation through advantageous properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


