用于抑制肿瘤转移、增强能量剥夺和免疫疗法的精密智能纳米导弹。
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
上皮-间质转化(EMT)、肿瘤基质和局部代谢改变共同建立了独特的肿瘤微环境(TME),促进了肿瘤的发展和转移。为应对这一挑战,我们设计了一种名为 HA@AT-Pd 的精准智能纳米导弹,可实现癌症相关成纤维细胞(CAF)转化和肿瘤细胞清除的双管齐下。研究发现,HA@AT-Pd 可通过阻断 TGF-β/Smad 信号通路介导的 EMT 抑制癌症干细胞(CSCs)的生成,并将活化的 CAFs 逆转至静止状态。值得注意的是,HA@AT-Pd通过同时抑制细胞氧化磷酸化和糖酵解,诱导乳腺癌细胞能量耗竭。糖酵解的抑制会减少乳酸的产生,从而将免疫抑制性 TME 转变为免疫激活环境。此外,HA@AT-Pd 产生的光热效应可诱发免疫性细胞死亡,从而进一步增强抗肿瘤免疫反应。总之,这种多功能组合策略揭示了抑制肿瘤进展和转移的潜在治疗途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
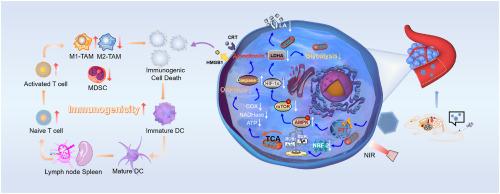
A precision intelligent nanomissile for inhibiting tumor metastasis, boosting energy deprivation and immunotherapy
The epithelial-mesenchymal transition (EMT), tumor stroma and local metabolic alterations cooperate to establish a unique tumor microenvironment (TME) that fosters tumor progression and metastasis. To tackle this challenge, a precision intelligent nanomissile named HA@AT-Pd has been designed for dual-pronged cancer-associated fibroblast (CAF) transformation and tumor cell elimination. It is observed that HA@AT-Pd inhibits the production of cancer stem cells (CSCs) by blocking the TGF-β/Smad signaling pathway-mediated EMT and reversing activated CAFs to quiescence. Notably, HA@AT-Pd induces energy depletion in breast cancer cells through simultaneously suppressing cellular oxidative phosphorylation and glycolysis. The inhibition of glycolysis results in reduced lactic acid production, thereby converting an immunosuppressive TME into an immune-activating environment. Furthermore, the photothermal effect generated by HA@AT-Pd evokes immunogenic cell death, which can further enhance the anti-tumor immune response. Overall, this multifunctional combination strategy unveils potential therapeutic avenues to inhibit tumor progression and metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


