二氧化碳/丁二烯衍生的三取代六元内酯的开环聚合反应
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
二氧化碳/丁二烯衍生内酯单体的选择性开环均聚已成为利用二氧化碳合成化学可回收聚酯的一种极具吸引力的方法。以往的研究仅集中于二取代六元内酯。在本研究中,使用 NaOMe、tBu-P4/BnOH 或 tBu-P4,通过选择性开环聚合(ROP)成功聚合了一种新设计的三取代六元二氧化碳/丁二烯衍生内酯单体--3,3,6-三乙基四氢-2H-吡喃-2-酮(Et-HL)。tBu-P4/BnOH 可生成具有典型活聚合行为的线性聚(Et-HL),而仅使用 tBu-P4 可使环状聚(Et-HL)达到 1050 kg mol-1 的最大数均分子量(Mn)和 1.52 的分散度(Đ)。为线性聚(Et-HL)和环状聚(Et-HL)的单体循环开发了催化方法。核磁共振(NMR)对关键中间产物的直接观察揭示了 Et-HL 和 HL 之间的机理差异。我们提出了一种从尾到头的应变释放机制,以合理解释仅使用 tBu-P4 就能选择性地形成 HL 和 Et-HL 的环状聚合物。就线性聚合物而言,HL 和 Et-HL 具有类似的机理,即引发剂阴离子攻击单体酯键。这项研究首次展示了含有 2 个以上取代基的六元内酯的 ROP,同时首次从根本上理解了 Thorpe-Ingold 效应对 CO2/丁二烯衍生六元内酯 ROP 的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
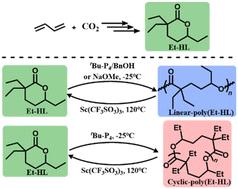
Ring-opening polymerization of a tri-substituted six-membered lactone derived from CO2/butadiene†
The selective ring-opening homo-polymerization of CO2/butadiene-derived lactone monomers has emerged as an appealing approach for synthesizing chemically recyclable polyesters from CO2. Previous research has only concentrated on CO2-derived di-substituted six-membered lactones. In this study, a newly designed tri-substituted six-membered CO2/butadiene-derived lactone monomer, 3,3,6-triethyltetrahydro-2H-pyran-2-one (Et-HL), was polymerized successfully through selective ring-opening polymerization (ROP) using NaOMe, tBu-P4/BnOH, or tBu-P4. tBu-P4/BnOH affords linear-poly(Et-HL) with typical living polymerization behaviors, while a maximum number-average molecular weight (Mn) of 1050 kg mol−1 and a dispersity (Đ) of 1.52 were achieved for cyclic-poly(Et-HL) using only tBu-P4. Catalytic methods were developed for monomer recycling of both linear- and cyclic-poly(Et-HL). Direct observation of key intermediates by Nuclear Magnetic Resonance (NMR) reveals the mechanistic differences between Et-HL and DEtP. A tail-to-head strain-releasing mechanism was proposed to rationalize the selective formation of cyclic polymers for both DEtP and Et-HL using only tBu-P4. In the case of linear polymers, DEtP and Et-HL share a similar mechanism involving the initiator anion attacking the monomer ester bond. This work represents the first example of the ROP of six-membered lactones bearing more than 2 substituents, simultaneously offering a fundamental understanding of the Thorpe–Ingold effect on the ROP of CO2/butadiene-derived six-membered lactones for the first time.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


