通过氮化碳上的活性氢调节实现大电流二氧化碳电甲烷化
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
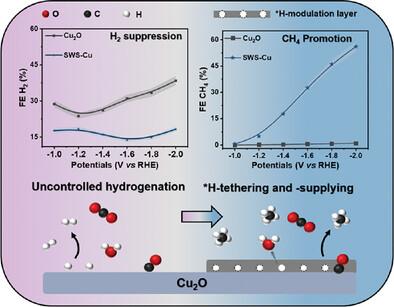
二氧化碳电甲烷化因其热值高、储存和运输方便以满足工业需求而受到广泛关注。然而,在高电流密度条件下,质子耦合电子转移动力学的多步骤缓慢和不期望的 *H 耦合限制了它的发展,给其商业化带来了巨大挑战。在这里,具有卓越氢吸附能力的氮化碳(CN)被用作活性氢吸附和供应材料。通过一种简便的液体辅助剥离和静电自组装策略,加强了其与 Cu2O 催化剂的界面接触,使 CH4 产率比原始 Cu2O 提高了 52 倍。流式细胞测试最终实现了 FECH4 和显著的 CH4 部分电流密度,分别为 61% 和 561 mA cm-2。通过原位 ATR-FTIR 光谱和 DFT 计算,可以确定强化的界面使 CN 纳米片中的 -C─N═C─ 位点拴住了丰富的 *H,并定向于 *CO 在 Cu 物种上氢化成 *CHO 和 *CHx。这项工作揭示了细扩展界面与尺寸材料对通过活性氢管理的产物分布和产量的深刻影响,这对其他以质子耦合电子转移(PCET)过程(如 N2、O2 等)为主的小分子电极化具有参考价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Large-Current CO2 Electromethanation Through Active Hydrogen Regulation Over Carbon Nitride
Electromethanation of CO2 has received intensive attention due to its high calorific value and convenient storage along with transportation to accommodate industrial demands. However, it is limited by sluggish multi-step proton-coupled electron transfer kinetics and undesired *H coupling under high current density, posing great challenges to its commercialization. Herein, carbon nitride (CN) with superior hydrogen adsorption ability is used as an active-hydrogen adsorption and supply material. Through a facile liquid-assisted exfoliation and electrostatic self-assembly strategy to strengthen its interfacial contacts with Cu2O catalysts, yielding a strengthened CH4 production 52 times higher than that of pristine Cu2O. Flow-cell test ultimately achieved FECH4 and remarkably CH4 partial current density of 61% and 561 mA cm−2, respectively. With in situ ATR-FTIR spectra and DFT calculations, it is established that strengthened interfaces enabled abundant *H tethered by ─C─N═C─ sites in CN nanosheets and oriented to the *CO hydrogenation to *CHO and *CHx on Cu species. This work reveals the profound influence of fine-expanded interfaces with dimensional materials on the product distribution and yield through the active-hydrogen management, which is of reference value for other small-molecule electro-polarization dominated by the proton-coupled electron transfer (PCET) process (e.g., N2, O2, etc.).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


