扩大可配体蛋白质组的化学工具:基于多样性合成的光活性立体配体
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
化学蛋白质组学可对原生生物系统中的小分子-蛋白质相互作用进行全面分析,已成为发现配体的一种多功能方法。然而,化学蛋白质组学探索的小分子范围仍然有限。在这里,我们描述了一个受多样性导向合成(DOS)启发的立体化学定义化合物库,其中含有重氮和炔烃单元,分别用于紫外光诱导的共价修饰和相互作用蛋白质的点击化学富集。我们发现,这些 "光立体探针 "能以立体选择性的方式与人体细胞中不同结构和功能类别的数百种蛋白质相互作用,并证明这些相互作用能为高通量筛选兼容的 NanoBRET 检测奠定基础。综合表型筛选和化学蛋白质组学发现了光立体探针,它们能通过与线粒体丝氨酸蛋白酶 CLPP 结合来调节自噬。我们的研究结果表明,DOS启发的光立体探针可用于扩展可配体蛋白质组、提供目标参与测定以及促进表型筛选中生物活性化合物的发现和表征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
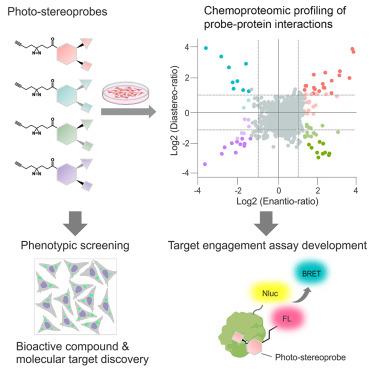

Chemical tools to expand the ligandable proteome: Diversity-oriented synthesis-based photoreactive stereoprobes
Chemical proteomics enables the global analysis of small molecule-protein interactions in native biological systems and has emerged as a versatile approach for ligand discovery. The range of small molecules explored by chemical proteomics has, however, remained limited. Here, we describe a diversity-oriented synthesis (DOS)-inspired library of stereochemically defined compounds bearing diazirine and alkyne units for UV light-induced covalent modification and click chemistry enrichment of interacting proteins, respectively. We find that these “photo-stereoprobes” interact in a stereoselective manner with hundreds of proteins from various structural and functional classes in human cells and demonstrate that these interactions can form the basis for high-throughput screening-compatible NanoBRET assays. Integrated phenotypic screening and chemical proteomics identified photo-stereoprobes that modulate autophagy by engaging the mitochondrial serine protease CLPP. Our findings show the utility of DOS-inspired photo-stereoprobes for expanding the ligandable proteome, furnishing target engagement assays, and facilitating the discovery and characterization of bioactive compounds in phenotypic screens.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


