构建掺铜的α-Fe2O3/γ-Fe2O3 异相结复合材料及其光催化性能
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
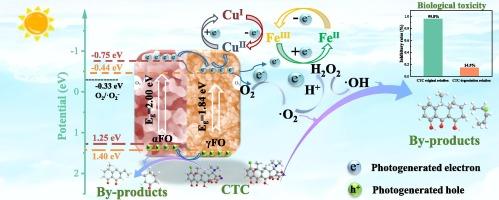
盐酸金霉素(CTC)是一类抗生素,对环境,尤其是水生生态系统造成严重危害。开发高效且可回收的水处理材料仍然是环境修复研究的一个重点。本研究采用溶热法和可控煅烧工艺制备了掺铜的α-Fe2O3/γ-Fe2O3异相结材料,以提高Fe2O3对水中四氯化碳的光催化降解能力。实验结果表明,该复合材料具有优异的磁性能,其中 Cu2-α-Fe2O3/γ-Fe2O3 具有高比表面积和小孔隙的特点,对四氯化碳的吸附效果最好。α-Fe2O3/γ-Fe2O3 的交错能级有利于电子快速转移,铜离子的加入优化了电子路径,产生了大量氧空位。这与异相结相结合,促进了电荷分离和迁移。在测试的样品中,Cu2-α-Fe2O3/γ-Fe2O3 复合材料具有最高效的光催化性能,在可见光照射下对四氯化碳的降解率达到 90.19%。所提出的二阶反应速率常数分别是 α-Fe2O3、γ-Fe2O3 和 α-Fe2O3/γ-Fe2O3 的约 31.99 倍、10.07 倍和 4.48 倍。该材料对其他抗生素(如土霉素、盐酸四环素等)也有良好的降解效果。此外,样品的结构形态在循环后保持稳定。在四氯化碳的光催化降解过程中,O2-和 h+ 是主要的活性物种,而 OH 起次要作用。通过使用密度函数理论(DFT)计算预测的自由基攻击位点,并分析四氯化碳降解过程的产物,阐明了可能的降解途径。此外,毒性风险评估表明,中间产物的毒性较低,对水生环境的潜在风险极小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Construction of Cu-doped α-Fe2O3/γ-Fe2O3 hetero-phase junction composite and its photocatalytic performance
Chlortetracycline hydrochloride (CTC), a class of antibiotics, poses a significant environmental hazard, particularly in aquatic ecosystems. The advancement of highly efficient and readily recyclable materials for water treatment remains an important focus of research in environmental remediation. In this study, Cu-doped α-Fe2O3/γ-Fe2O3 hetero-phase junction materials were prepared by a solvothermal method and a controlled calcination process to enhance the photocatalytic degradation of CTC in water by Fe2O3. Experimental results indicate that the composite material has superior magnetic properties, with Cu2-α-Fe2O3/γ-Fe2O3, featuring a high specific surface area and small pores, showing the best CTC adsorption. The α-Fe2O3/γ-Fe2O3′s interlaced energy levels facilitate quick electron transfer, and copper ion addition optimizes electron paths, generating numerous oxygen vacancies. This, combined with the hetero-phase junction, boosts charge separation and migration. Among the samples tested, The Cu2-α-Fe2O3/γ-Fe2O3 composite demonstrated the most efficient photocatalytic performance, with a degradation rate of 90.19 % for CTC achieved under Visible Light Irradiation. The proposed second-order reaction rate constants were approximately 31.99, 10.07, and 4.48 times higher than those for α-Fe2O3, γ-Fe2O3, and α-Fe2O3/γ-Fe2O3, respectively. The material also demonstrates good degradation effects on other antibiotics (such as oxytetracycline, tetracycline hydrochloride, etc.). Moreover, the structural morphology of the sample remains stable after cycling.  O2– and h+ are the main active species in the photocatalytic degradation process of CTC, while
O2– and h+ are the main active species in the photocatalytic degradation process of CTC, while  OH plays a secondary role. The possible degradation pathways were elucidated by calculating predicted free radical attack sites using density-functional theory (DFT) and by analyzing the products of the CTC degradation process. Additionally, the toxicity risk assessment indicates that the intermediate products have low toxicity and pose a minimal potential risk to the aquatic environment.
OH plays a secondary role. The possible degradation pathways were elucidated by calculating predicted free radical attack sites using density-functional theory (DFT) and by analyzing the products of the CTC degradation process. Additionally, the toxicity risk assessment indicates that the intermediate products have low toxicity and pose a minimal potential risk to the aquatic environment.
 O2– and h+ are the main active species in the photocatalytic degradation process of CTC, while
O2– and h+ are the main active species in the photocatalytic degradation process of CTC, while  OH plays a secondary role. The possible degradation pathways were elucidated by calculating predicted free radical attack sites using density-functional theory (DFT) and by analyzing the products of the CTC degradation process. Additionally, the toxicity risk assessment indicates that the intermediate products have low toxicity and pose a minimal potential risk to the aquatic environment.
OH plays a secondary role. The possible degradation pathways were elucidated by calculating predicted free radical attack sites using density-functional theory (DFT) and by analyzing the products of the CTC degradation process. Additionally, the toxicity risk assessment indicates that the intermediate products have low toxicity and pose a minimal potential risk to the aquatic environment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


