通过氮化碳中的碳空位调节单原子锰区的电荷密度,高效激活高碘酸盐降解磺胺甲噁唑
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
涉及高碘酸盐(PI)的高级氧化系统已经证明能够有效降解水中的某些有机污染物。该过程的一个关键方面是高碘酸盐的有效活化。在本实验中,通过简单的高温煅烧方法制备了含有碳空位的石墨氮化碳(Cv-CN),并在其表面均匀地分散了锰原子,形成了 Mn-Cv-CN。碳空位的存在调节了单原子锰周围的电子密度,显著提高了 PI 的活化效率。氮化碳/碘酸根(Mn-Cv-CN/PI)体系中表面锚定的锰原子与碳空位在 25 分钟内降解了 99% 的磺胺甲噁唑(SMX)。检测到了 SMX 的各种降解因子,包括 O2-、1O2、IO3、OH 和电子传递过程,其中 O2-被确定为主要的活性氧(ROS)。PI 通过从活性位点获得电子而被激活,产生大量的 O2- 来催化转化污染物。液相色谱-质谱法用于分析 SMX 可能的降解途径和中间产物。多项实验表明,Mn-Cv-CN/PI 系统在处理含有难降解抗生素的水体方面具有良好的潜力。研究结果为利用单原子催化剂高效活化 PI 以去除水中难降解污染物提供了一种新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。

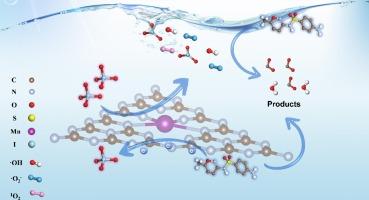
Efficient activation of periodate for sulfamethoxazole degradation by modulating charge density in single-atom Mn regions via carbon vacancies in carbon nitride
The advanced oxidation system involving periodate (PI) has demonstrated effective degradation capabilities for certain organic pollutants in water. A critical aspect of this process is the efficient activation of periodate. In this experiment, carbon vacancy-containing graphitic carbon nitride (Cv-CN) was prepared through a simple high-temperature calcination method, and Mn atoms were uniformly dispersed on its surface, forming Mn-Cv-CN. The presence of carbon vacancies modulated the electron density around the single-atom Mn, significantly enhancing the activation efficiency of PI. Surface-anchored Mn atoms with carbon vacancies in carbon nitride/periodate (Mn-Cv-CN/PI) system degraded 99% of sulfamethoxazole (SMX) within 25 min. Various degradation factors for SMX, including ![]() O2−, 1O2,
O2−, 1O2, ![]() IO3,
IO3, ![]() OH, and electron transfer processes, were detected, with
OH, and electron transfer processes, were detected, with ![]() O2− identified as the primary reactive oxygen species (ROS). PI was activated by acquiring electrons from active sites, generating a substantial amount of
O2− identified as the primary reactive oxygen species (ROS). PI was activated by acquiring electrons from active sites, generating a substantial amount of ![]() O2− to catalytically transform the pollutants. Liquid chromatography-mass spectrometry was used to analyze the possible degradation pathways and intermediates of SMX. Multiple experiments demonstrated that the Mn-Cv-CN/PI system showed promising potential in treating water containing recalcitrant antibiotics. The findings provided a novel strategy for efficiently activating PI with single-atom catalysts to remove refractory pollutants from water.
O2− to catalytically transform the pollutants. Liquid chromatography-mass spectrometry was used to analyze the possible degradation pathways and intermediates of SMX. Multiple experiments demonstrated that the Mn-Cv-CN/PI system showed promising potential in treating water containing recalcitrant antibiotics. The findings provided a novel strategy for efficiently activating PI with single-atom catalysts to remove refractory pollutants from water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


