抗菌生物适应性支架通过内在刺激和免疫调节活性的协同作用促进血管化骨再生
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
血管生成和骨生成的耦合是骨骨折愈合的基本需要,因此组织工程骨的模拟作用在骨缺损治疗中具有强大而有益的效果。本文基于聚电解质修饰的三维打印支架,构建了一种具有血管生成和成骨耦合内在活性的免疫调节生物复合支架,用于增强骨再生。在三维打印聚己内酯(PCL)支架中掺入掺杂锶的羟基磷灰石(SrHA),然后通过羧甲基壳聚糖(CCS)/超支化聚赖氨酸(HBPL)表面涂层吸附缝隙引导配体3(SLIT3)蛋白,实现了SLIT3和锶离子的按需输送。聚电解质修饰支架的抗菌性能与聚电解质涂层的层数成正比。该双因子递送支架具有良好的生物相容性,支持细胞增殖和迁移,并能通过释放的 SLIT3 蛋白和锶离子的内在激励刺激血管生成和骨生成。重要的是,该多功能支架具有免疫调节作用,可促进巨噬细胞的 M2 型极化,从而提高抗炎因子水平,并间接促进血管生成和骨生成。体内实验显示,植入多功能支架后,抗炎效果明显增强,提供了更好的再生微环境,骨再生能力显著提高,同时形成了 H 型血管。因此,该生物适应性支架通过内在激励和免疫调节作用提高了骨再生性能,有望成为骨缺损修复的治疗候选材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
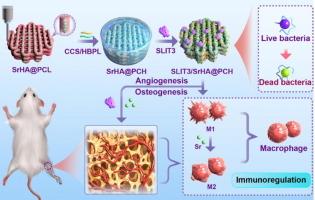
Antibacterial bioadaptive scaffold promotes vascularized bone regeneration by synergistical action of intrinsic stimulation and immunomodulatory activity
The coupling of angiogenesis and osteogenesis is the fundamental necessity in bone fracture healing, thus simulative action of tissue-engineered bone provides powerful and beneficial effects in the treatment of bone defect. Herein, an immunoregulatory biocomposite scaffold with intrinsic activities of coupling angiogenesis and osteogenesis was constructed based on polyelectrolytes-modified 3D-printed scaffold for enhanced bone regeneration. The doping of Sr-doped hydroxyapatite (SrHA) within 3D-printed polycaprolactone (PCL) scaffold and subsequent slit guidance ligand 3 (SLIT3) protein adsorption through surface coating of carboxymethyl chitosan (CCS)/hyperbranched polylysine (HBPL) achieved on-demand delivery of SLIT3 and Sr ions. The antibacterial property of polyelectrolytes-modified scaffold was characterized and was directly proportional to the layer number of polyelectrolytes coating. The dual-factor delivery scaffold had good biocompatibility to support cell proliferation and migration, and was capable of stimulating angiogenesis and osteogenesis by intrinsic stimulation from released SLIT3 protein and Sr ions. Importantly, the multifunctional scaffold had immunomodulatory effects of promoting M2-type polarization of macrophages and thereby increasing anti-inflammatory factors level, as well as indirectly promoting angiogenesis and osteogenesis. The in vivo experiments revealed that the anti-inflammatory effect was significantly reinforced for providing a better regenerative microenvironment and bone regeneration capacity was dramatically enhanced accompanied with type H vessels formation when implanted with multifunctional scaffold. Therefore, the bioadaptive scaffold possessed amplified bone regeneration performance through intrinsic stimulation and immunomodulatory effects, suggesting a promising therapeutic candidate for bone defect repair.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


