通过硫化改善纳米级零价铁的厌氧腐蚀和去污关系的动力学见解
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
纳米级零价铁(nZVI)的腐蚀和去污之间存在权衡,而硫化可对其进行调节。尽管如此,从动力学角度来看,对硫酸化 nZVI(S-nZVI)的厌氧腐蚀与去污之间关系的认识仍不清楚。在此,我们利用开路电位的变化来探索 S-nZVI 的厌氧腐蚀动力学。以六价铬为目标污染物,发现在反应的前 10 分钟(R2 = 0.6919,斜率 = -0.7091)和 10 分钟后(R2 = 0.7556,斜率 = -0.1307),去污与 S-nZVI 的腐蚀之间存在负相关。根据 TEM 图谱、电化学阻抗光谱和 XPS 的结果,本研究进一步揭示了硫化不仅在反应初期增强了 Cr(VI) 向 nZVI 的质量转移,而且在反应后期改善了 nZVI 向 Cr(VI) 的电子转移。S-nZVI 最终增强了对六价铬的去除和还原,这应归功于硫化铁(FeSx)的引入,它不仅提高了导电性,还有利于 nZVI 对六价铬的亲和性。总之,本研究提出的动力学见解对于理解 S-nZVI 在厌氧水中的过程机制很有价值,并可为开发使用 nZVI 修复受污染地下水的强化方法提供理论支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
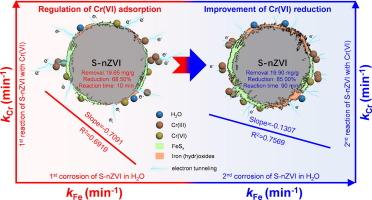
Kinetic insights into the relationships between anaerobic corrosion and decontamination of nanoscale zerovalent iron improved by sulfidation
There is a trade-off between corrosion and decontamination of nanoscale zero-valent iron (nZVI), which can be modulated by the sulfidation. Despite that, the insights into the relationship between anaerobic corrosion and decontamination of sulfidated nZVI (S-nZVI) remain unclear from a kinetic point of view. Herein, we used the variation of open-circuit potential to explore the anaerobic corrosion kinetic of S-nZVI. Taking Cr(VI) as the targeted contaminant, a negative correlation between decontamination and the corrosion of S-nZVI was identified during the first 10 min of reaction (R2 = 0.6919, slope = -0.7091) and after the 10 min of reaction (R2 = 0.7556, slope = -0.1307). Based on the results of TEM mapping, electrochemical impedance spectroscopy and XPS, this study further revealed that sulfidation not only enhanced the mass transfer of Cr(VI) toward nZVI in the initial stage of reaction, but also improved the electron transfer of nZVI toward Cr(VI) in the later stage of reaction. The ultimately enhanced removal and reduction of Cr(VI) by S-nZVI should be attributed to the introduction of iron sulfides (FeSx) that not only promoted the conductivity but also favored the affinity of nZVI toward Cr(VI). Overall, the kinetic insights presented in this study are valuable for understanding the process mechanisms of S-nZVI in anaerobic water and may extend to provide theoretical support for the development of enhanced methods for the remediation of contaminated groundwater using nZVI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


