掺杂 Sr2+ 对六方包晶 Ba7-xSrxNb4MoO20-δ 电解质结构和电学特性的影响:实验和 DFT 建模研究
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
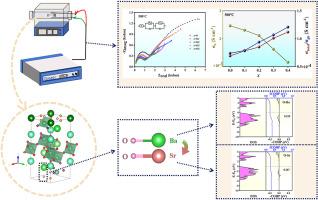
Ba7Nb4MoO20 具有优异的氧离子传输特性,是一种很有前途的固体氧化物燃料电池(SOFC)电解质材料。为了进一步提高其氧离子传导性,元素掺杂是一种有效的策略。然而,很少有研究从电子结构的角度深入探讨掺杂策略对电解质导电性能的影响机制。本文采用状态密度(DOS)和晶体轨道汉密尔顿群(COHP)的方法分析了 Ba7Nb4MoO20 中氧离子电导率的增强机制。由于电解质的电子电导率可以忽略不计,因此它们的总电导率基本上可以看作是氧离子的电导率。随着锶掺杂量的增加,电解质的氧离子电导率也随之增加。由于 Sr-O 的键能比 Ba-O 高,因此体电导率与 Sr 掺杂量呈负相关。另一方面,Sr 会促进晶粒生长并减少晶界数量,从而降低晶界的氧扩散阻力,进而提高晶界电导率。在 Ba7-xSrxNb4MoO20-δ(x=0、0.1、0.2、0.3 和 0.4)包晶氧化物中,Ba6.6Sr0.4Nb4MoO20-δ 的导电率最高,在 500°C 时达到 1.12 × 10-4S cm-1。这项研究不仅开发出了一种具有广阔应用前景的 SOFC 电解质材料,还为其掺杂改性提供了理论指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effect of Sr2+ doping on the structure and electrical properties of hexagonal perovskite Ba7-xSrxNb4MoO20-δ electrolyte: experimental and DFT modeling studies
Ba7Nb4MoO20 exhibits excellent oxygen ion transport properties and is a promising electrolyte material for solid oxide fuel cell (SOFC). To further enhance its oxygen ionic conductivity, element doping is an effective strategy. However, few studies have delved into the impact mechanism of doping strategies on the electrolyte's conductivity properties from the perspective of electronic structure. Here, the enhancement mechanism of oxygen ionic conductivity in Ba7Nb4MoO20 was analyzed using the the methods of density of states (DOS) and Crystal Orbital Hamilton Populations (COHP). Since the electronic conductivities of the electrolytes are negligible, their total conductivies can essentially be regarded as the conductivies of oxygen ions. As the Sr doping amount increases, the oxygen ionic conductivities of the electrolytes also increase. The bulk conductivity shows a negative correlation with the Sr doping amount, which is due to the higher bond energy of Sr-O compared to Ba-O. On the other hand, Sr promotes grain growth and reduces the number of grain boundaries, thereby decreasing the resistance to oxygen diffusion at the grain boundaries and thus enhancing the grain boundary conductivity. In the Ba7-xSrxNb4MoO20-δ (x=0, 0.1, 0.2, 0.3, and 0.4) perovskite oxides, Ba6.6Sr0.4Nb4MoO20-δ has the highest conductivity, reaching 1.12 × 10-4S cm-1 at 500°C. This work not only develops a SOFC electrolyte material with promising application prospects, but also provides theoretical guidance for its doping modification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


