利用硅笼中的过渡金属定制超原子稳定性:收缩至 M@Si15(M = Re、Os、Ir)
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
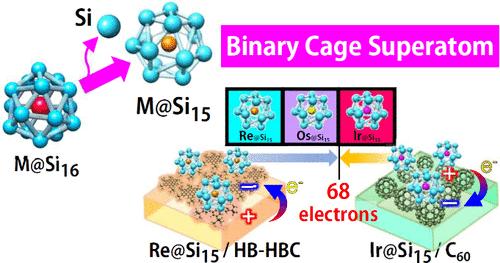
设计具有迷人电子特性的材料对于推动纳米技术的发展至关重要,因为在纳米技术中,对原子结构和电子行为的精确控制至关重要。金属封装硅笼超原子(SAs)为分子尺度材料设计提供了一种新的范例,允许对结构和电子特性进行微调。模拟卤素、惰性气体和碱金属的超原子的形成已得到深入研究,特别是 M@Si16,其中第 3 至第 5 族的早期过渡金属稳定在 Si16 笼中,实现了 68 电子构型。对于电子过剩的晚期过渡金属来说,Si15笼通过减少一个Si原子来满足68电子规则,从而增强了稳定性。这项研究在 p 型和 n 型有机基底上合成了含有 7 族铼(Re)和 9 族铱(Ir)的 Si15 笼状砷化镓。通过 X 射线光电子能谱和理论计算评估了 Re@Si15 和 Ir@Si15 的氧化反应稳定性,其中包括第 8 族的锇(Os)。其中,P 型有机基底上的 Re@Si15- 显示出卓越的电子和几何特性。这些发现增进了我们对过渡金属 M@Sin 系统的了解,解决了有关它们在 n = 15 和 16 时的性质的长期问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tailoring Superatomic Stability with Transition Metals in Silicon Cages: Shrinking to M@Si15 (M = Re, Os, Ir)
The design of materials with intriguing electronic properties is crucial for advancing nanoscale technologies, where precise control over atomic structure and electronic behavior is essential. Metal-encapsulating silicon cage superatoms (SAs) provide a new paradigm for molecular-scale material design, allowing fine-tuning of both structure and electronic characteristics. The formation of superatoms mimicking halogens, noble gases, and alkali metals has been well-studied, particularly with M@Si16, where early transition metals from groups 3 to 5 stabilize within a Si16 cage, achieving a 68-electron configuration. For late transition metals with excess electrons, a Si15 cage offers enhanced stability by fulfilling the 68-electron rule with one fewer Si atom. This research synthesizes Si15 cage-SAs with rhenium (Re) from group 7 and iridium (Ir) from group 9 on p-type and n-type organic substrates. The stability of Re@Si15 and Ir@Si15 is evaluated via oxidative reactivity with X-ray photoelectron spectroscopy and theoretical calculations, including osmium (Os) from group 8. Re@Si15–, Os@Si150, and Ir@Si15+ exhibit superatomic behaviors similar to halogens, noble gases, and alkali metals due to the 68-electron shell closure. Among them, Re@Si15– on p-type organic substrates shows superior electronic and geometric properties. These findings advance our understanding of M@Sin systems for transition metals, addressing longstanding questions about their properties at n = 15 and 16.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


