髓系 NPC1 缺乏会损害巨噬细胞的排泄功能,从而加剧肝损伤和肝纤维化
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
导言尼曼-皮克 C1(NPC1)是一种溶酶体胆固醇转运蛋白,需要有效的排泄功能。我们的研究旨在确定髓系细胞 NPC1 在肝损伤中的功能作用,并阐明其潜在机制。方法:对肝病患者的损伤肝脏和小鼠模型进行分析,以检测 NPC1 的表达。为确定巨噬细胞 NPC1 在肝损伤中的功能作用,构建了髓细胞特异性 Npc1 基因敲除小鼠。结果我们发现 NPC1 主要在肝巨噬细胞中表达。在患者和小鼠模型的损伤肝脏中,其 mRNA 和蛋白表达均显著升高。在注射四氯化碳(CCl4)和蛋氨酸胆碱缺乏(MCD)饮食诱导的肝损伤小鼠模型中,组织特异性缺失髓系 Npc1 会增加肝脏炎症、血清肝功能酶水平和肝纤维化。进一步的分析表明,在肝损伤小鼠模型中缺乏 Npc1 会导致血清 HMGB1 和线粒体 DNA 水平升高,促进肝巨噬细胞促炎性活化、M1 极化,导致肝脏炎性细胞因子/凝血因子(如 CCL2)过度产生和 STING/NFκB 通路活化。体外机制研究显示,Npc1缺陷的巨噬细胞表现出低效的排泄功能,部分原因是货物降解功能受损。NPC1的功能障碍会加重肝损伤,这表明NPC1可能是治疗肝病的潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
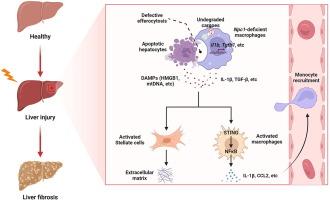
Deficiency of myeloid NPC1 exacerbates liver injury and fibrosis by impairing macrophage efferocytosis
Introduction
Niemann-Pick C1 (NPC1), a lysosomal cholesterol transport protein, is required for efficient efferocytosis. Patients with Npc1 mutation are frequently accompanied with hepatic symptoms, including hepatomegaly, elevated liver transaminases, or even acute liver failure, but the pathogenic mechanism remains unknown.Objectives
Our work aims to characterize the functional role of myeloid NPC1 in liver injury and elucidate its underlying mechanism.Methods
Analyses of injured livers from patients with liver diseases and mouse models were conducted to examine NPC1 expression. Myeloid cell-specific Npc1 knockout mice were constructed to determine the functional role of macrophage NPC1 in liver injury. Isolated macrophages were subjected to in vitro mechanistical assays.Results
We found that NPC1 is mainly expressed in hepatic macrophages. Its mRNA and protein expression are significantly elevated in injured livers from both patients and mouse models. Tissue-specific deletion of myeloid Npc1 increased liver inflammation, levels of serum liver function enzymes, and liver fibrosis in mouse models of liver injury induced by carbon tetrachloride (CCl4) injection and methionine-and-choline-deficient (MCD) diets. Further analyses indicate that Npc1 deficiency in mouse models of liver injury resulted in increased levels of serum HMGB1 and mitochondrial DNA, promoted hepatic macrophage proinflammatory activation, M1 polarization, led to overproduction of hepatic inflammatory cytokines/chemokines, e.g. CCL2 and STING/NFκB pathway activation. In vitro mechanistical studies reveal that Npc1-deficient macrophages exhibited inefficient efferocytosis, partly due to impaired cargo degradation.Conclusions
These findings indicate that elevated expression of myeloid NPC1 in liver diseases protects liver from injury by promoting macrophage efferocytosis of damaged cells. Dysfunction of NPC1 aggravates liver injury, suggesting that NPC1 may be a potential therapeutic target for treating liver diseases.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


