致癌胆固醇通过 CSNK2A1-IGF2R Ser2484 轴重构肝细胞癌中的脂质代谢
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
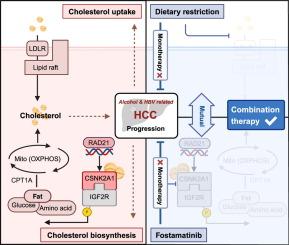
导言:饮酒和乙型肝炎病毒(HBV)感染是肝细胞癌(HCC)的常见危险因素。方法纳入两组独立的 HCC 患者(n = 539 和 n = 140),研究具有协同酒精和 HBV(AB-HCC)背景的 HCC。患者衍生细胞系、器官组织和异种移植物被用来验证脆弱的新陈代谢。结果在这里,我们通过整合临床队列、蛋白质组学、磷酸化蛋白质组学和空间转录组,将 AB-HCC 划分为以致癌胆固醇为特征的独特代谢亚型。从机理上讲,我们的研究结果表明,胆固醇会直接与 CSNK2A1(酪蛋白激酶 2 Alpha 1)结合,增强其激酶活性,导致 IGF2R(胰岛素样生长因子 2 受体)在 Ser2484 处磷酸化。这种级联反应重新连接了脂质驱动的线粒体氧化磷酸化,产生了丙二醛测定法测量的活性氧,并使胆固醇生物合成的正反馈循环得以延续,最终导致肿瘤发生。在 AB-HCC 中,CSNK2A1 最初的转录激活是由 RAD21 的上调驱动的。我们的胆固醇分析揭示了AB-HCC的代偿机制,即利用胆固醇的摄取和生物合成来获得生存优势。此外,高通量药物筛选和体内验证发现了AB-HCC的易感性,通过限制饮食中胆固醇摄入和口服福斯他替尼可以有效解决这一问题。CSNK2A1介导的胆固醇生物合成途径与多种以胆固醇代谢为特征的癌症有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Oncogenic cholesterol rewires lipid metabolism in hepatocellular carcinoma via the CSNK2A1-IGF2R Ser2484 axis
Introduction
Alcohol consumption and hepatitis B virus (HBV) infection are common risk factors for hepatocellular carcinoma (HCC). However, few studies have focused on elucidating the mechanisms of HCC with combined alcohol and HBV etiology.
Objectives
We aimed to investigate the molecular features of alcohol and HBV on HCC and to seek out potential therapeutic strategies.
Methods
Two independent cohorts of HCC patients (n = 539 and n = 140) were included to investigate HCC with synergetic alcohol and HBV (AB-HCC) background. Patient-derived cell lines, organoids, and xenografts were used to validate the metabolic fragile. High-throughput drug screening (1181 FDA-approved anticancer drugs) was leveraged to explore the potential therapeutic agents.
Results
Here, we delineated AB-HCC as a distinctive metabolic subtype, hallmarked by oncogenic cholesterol, through the integration of clinical cohorts, proteomics, phosphoproteomics, and spatial transcriptome. Mechanistically, our findings revealed that cholesterol directly binds to CSNK2A1 (Casein Kinase 2 Alpha 1), augmenting its kinase activity and leading to phosphorylation of IGF2R (Insulin-Like Growth Factor 2 Receptor) at Ser2484. This cascade rewires lipid-driven mitochondrial oxidative phosphorylation, spawns reactive oxygen species measured by malondialdehyde assay, and perpetuates a positive feedback loop for cholesterol biosynthesis, ultimately culminating in tumorigenesis. Initial transcriptional activation of CSNK2A1 is driven by upregulation of RAD21 in AB-HCC. Our cholesterol profiling exposes AB-HCC’s compensatory mechanism of AB-HCC, which capitalizes on both uptake and biosynthesis of cholesterol to confer survival edge. Moreover, high-throughput drug screening coupled with in vivo validation has uncovered the susceptibilities of AB-HCC, which can be effectively addressed by a combination of dietary cholesterol restriction and oral administration of Fostamatinib. The CSNK2A1-mediated cholesterol biosynthesis pathway has been implicated in various cancers characterized by cholesterol metabolism.
Conclusion
These findings not only pinpoint the oncogenic metabolite cholesterol as a hidden culprit in AB-HCC subtype, but also enlighten a novel combination strategy to rejuvenate tumor metabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


