用于癌症治疗的具有增强树突状细胞成熟功能的 pH 和氧化还原双响应纳米粒子
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
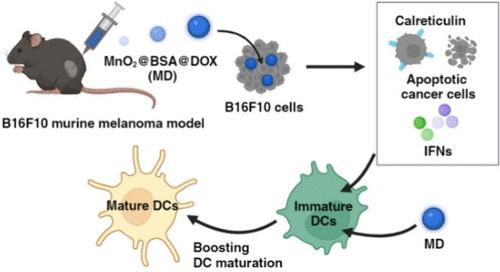
I 型干扰素(IFNs)对于激活树突状细胞(DCs)和向 T 细胞展示肿瘤相关抗原至关重要。IFNs 主要由免疫细胞中的 DCs 产生。化疗与金属免疫疗法相结合,可通过激活环GMP-AMP合成酶-干扰素基因刺激器(cGAS-STING)途径诱导IFN的产生。然而,尽管化疗药物具有强大的抗癌活性,但它们会消耗直流电群,抑制免疫刺激活性。此外,化疗药物与金属之间激活 DC 的最佳比例尚未报道,也缺乏确保 DC 存活的证据。在本研究中,我们假设存在一种最佳比例,可以在最小的直流细胞耗竭的情况下产生最高的直流细胞成熟度和抗癌活性。为了证明这一点,我们设计了一种 pH 和氧化还原双响应纳米粒子 MnO2@BSA@DOX(MD),以防止 DC 消耗并激活癌细胞和 DC 中的 cGAS-STING 通路,诱导相当水平的 IFNs 和成熟。MD 由核心层结构、二氧化锰(MnO2)核心和牛血清白蛋白(BSA)与多柔比星(DOX)交联层组成,DOX 与锰的比例特定。MD 针对肿瘤微环境的细胞外 pH 值和癌细胞的细胞内氧化还原反应,在癌细胞和直流细胞之间表现出基于结构的选择性。在各种配方中,1:1 的比例显示出最高的成熟度,且没有明显的消耗。此外,它还能通过细胞凋亡和 cGAS-STING 激活诱导癌细胞产生明显的细胞毒性,从而导致钙网蛋白表达增加和 DC 吞噬作用增强。因此,MD 在肿瘤中的高积累性和未观察到的全身毒性使其具有卓越的肿瘤抑制作用和延长生存期的效果,从而凸显了其作为癌症治疗剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
pH and Redox Dual-Responsive Nanoparticle with Enhanced Dendritic Cell Maturation for Cancer Therapy
Type I interferons (IFNs) are essential for activating dendritic cells (DCs) and presenting tumor-associated antigens to T cells. IFNs are primarily produced from DCs among immune cells. A combination of chemotherapy and metalloimmunotherapy induces IFN production by activating the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway. However, chemotherapeutic agents deplete DC populations, suppressing immunostimulatory activities, despite their potent anticancer activities. Furthermore, an optimal ratio between chemotherapeutic agents and metal for activating DCs at the highest level has not been reported, and evidence for ensuring DC survival is lacking. In this study, we hypothesized that there is an optimal ratio to yield the highest DC maturation and anticancer activity with minimal DC depletion. To demonstrate it, we have designed a pH and redox dual-responsive nanoparticle, MnO2@BSA@DOX (MD), to prevent DCs from depleting and activate the cGAS-STING pathway both in cancer cells and DCs, inducing considerable levels of IFNs and maturation. MD consists of a core-layer structure, a manganese dioxide (MnO2) core, and a cross-linked layer with bovine serum albumin (BSA) and doxorubicin (DOX), with a specific ratio of DOX to manganese. MD exhibits structure-based selectivity between cancer cells and DCs by targeting the extracellular pH of the tumor microenvironment and intracellular redox reactions in cancer cells. Among various formulations, the 1:1 ratio shows the highest maturation with no significant depletion. Moreover, it induces distinct cytotoxicity in cancer cells through apoptosis and cGAS-STING activation, leading to increased calreticulin expression and enhanced DC phagocytosis. Consequently, it results in superior tumor suppression and prolonged survival with the high accumulation of MD in the tumor and no observed systemic toxicities, highlighting its potential as a therapeutic agent in cancer treatments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


