通过甲烷的阳极氧化作用提高 SOEC 在 850 ℃、0.46 V @ 0.4 A/cm2 低电压下的制氢能力
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
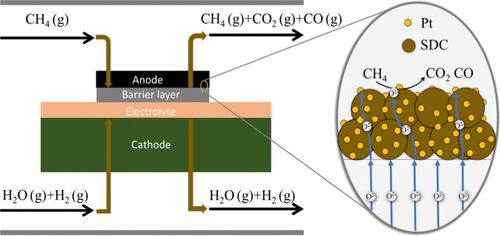
在固体氧化物电解槽的阳极侧引入甲烷可以在低电压下消耗氧气并产生氢气。本研究采用掺杂-分离法获得了铂负载的 Sm0.2Ce0.8O1.9 催化剂(Pt-SDC),该催化剂具有优异的甲烷氧化催化活性。结果表明,4%的 Pt-SDC 在 0.46 V 的电压下,阳极上甲烷氧化的电流密度高达 0.4 A/cm2,甲烷氧化的活化能相对较低,为 80.19 kJ/mol。电化学性能在很大程度上取决于热氧化活性。阻抗拟合显示,纯 SDC 的极化电阻从 3.27 Ω cm2 降至 4% Pt-SDC 的 0.73 Ω cm2。结果表明,甲烷在阳极一侧被氧化。铂的加入增强了电解池的稳定性,4% Pt-SDC 和 Pure SDC 的电压上升率分别为 0.2 mV/h和 1.8 mV/h。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydrogen Production at a Low Voltage of 0.46 V @ 0.4 A/cm2 at 850 °C in SOECs Enhanced by Anode Oxidation of Methane
Introducing methane on the anode side of solid oxide electrolysis cells can consume oxygen species and produce hydrogen at a low voltage. This study employs an incorporation–segregation method to obtain platinum loaded Sm0.2Ce0.8O1.9 catalysts (Pt-SDC) with excellent catalytic activity for methane oxidation. The results show that 4% Pt-SDC achieves a high current density of 0.4 A/cm2 at 0.46 V with methane oxidation over the anode, which exhibits a relatively low activation energy of 80.19 kJ/mol for methane oxidation. The electrochemical performance is highly dependent on thermal oxidation activity. Impedance fitting reveals a reduced polarization resistance from 3.27 Ω cm2 for Pure SDC to 0.73 Ω cm2 for 4% Pt-SDC. The results indicate that methane is oxidized on the anode side. The incorporation of Pt enhances the stability of the electrolysis cell, and the voltage increase rate is 0.2 mV/h for 4% Pt-SDC and 1.8 mV/h for Pure SDC, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


