氟伏沙明对Sigma-1受体的调节可改善丙戊酸诱导的大鼠自闭症行为:慢性ER应激调节、自噬增强和M1/M2小胶质细胞极化的参与。
IF 5.3
2区 医学
Q1 CLINICAL NEUROLOGY
Progress in Neuro-Psychopharmacology & Biological Psychiatry
Pub Date : 2024-11-05
DOI:10.1016/j.pnpbp.2024.111192
引用次数: 0
摘要
自闭症谱系障碍(ASD)是一种神经发育障碍。氟伏沙明(FVX)是一种抗抑郁药,广泛用于自闭症谱系障碍患者,但临床结果尚无定论,FVX治疗自闭症谱系障碍的机制也不清楚。本研究确定了σ-1受体(S1R)激动剂FVX对丙戊酸(VPA)诱导的自闭症模型的潜在治疗作用。在妊娠第 12.5 天,给 Wistar 怀孕大鼠腹腔注射一次 VPA(600 毫克/千克)或生理盐水(10 毫升/千克,载体对照)。从出生后第 21 天到第 50 天,给雄性幼崽注射 FVX(30 毫克/千克,每天一次)和 NE-100(S1R)拮抗剂(1 毫克/千克,每天一次)。实验结束时对行为测试和组织病理学变化进行鉴定。此外,还评估了小脑内质网(ER)应激和自噬的生物标志物。还评估了小胶质细胞极化为M1和M2表型的情况。FVX能有效缓解VPA引起的小脑组织病理学改变。除了对自噬增强、ER应激恶化和控制小胶质细胞极化有显著影响外,FVX还增强了交际能力和刻板行为。目前的研究证实,S1R激动剂FVX可以减轻VPA诱导的自闭症大鼠模型的行为和神经化学改变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
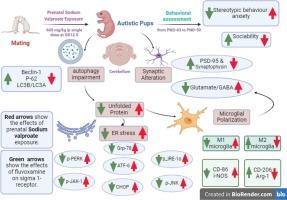
Sigma-1 receptor modulation by fluvoxamine ameliorates valproic acid-induced autistic behavior in rats: Involvement of chronic ER stress modulation, enhanced autophagy and M1/M2 microglia polarization
Autism spectrum disorder (ASD) is a neurodevelopmental disorder. While, fluvoxamine (FVX) is an antidepressant and widely prescribed to ASD patients, clinical results are inconclusive and the mechanism of FVX in the management of ASD is unclear. This study determined the potential therapeutic impact of FVX, a sigma-1 receptor (S1R) agonist, against the valproic acid (VPA)-induced model of autism. On gestational day 12.5, Wistar pregnant rats were given a single intraperitoneal (i.p.) injection of either VPA (600 mg/kg) or normal saline (10 mL/kg, vehicle-control). Starting on postnatal day (PND) 21 to PND 50, FVX (30 mg/kg, P·O. daily) and NE-100, (S1R) antagonist, (1 mg/kg, i.p. daily) were given to male pups. Behavior tests and histopathological changes were identified at the end of the experiment. In addition, the cerebellum biomarkers of endoplasmic reticulum (ER) stress and autophagy were assessed. Microglial cell polarization to M1 and M2 phenotypes was also assessed. FVX effectively mitigated the histopathological alterations in the cerebellum caused by VPA. FVX enhanced sociability and stereotypic behaviors in addition to its noteworthy impact on autophagy enhancement, ER stress deterioration, and controlling microglial cell polarization. The current investigation confirmed that the S1R agonist, FVX, can lessen behavioral and neurochemical alterations in the VPA-induced rat model of autism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
12.00
自引率
1.80%
发文量
153
审稿时长
56 days
期刊介绍:
Progress in Neuro-Psychopharmacology & Biological Psychiatry is an international and multidisciplinary journal which aims to ensure the rapid publication of authoritative reviews and research papers dealing with experimental and clinical aspects of neuro-psychopharmacology and biological psychiatry. Issues of the journal are regularly devoted wholly in or in part to a topical subject.
Progress in Neuro-Psychopharmacology & Biological Psychiatry does not publish work on the actions of biological extracts unless the pharmacological active molecular substrate and/or specific receptor binding properties of the extract compounds are elucidated.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


