用于儿科医院的可穿戴生物传感器:范围综述。
IF 3.1
3区 医学
Q1 PEDIATRICS
引用次数: 0
摘要
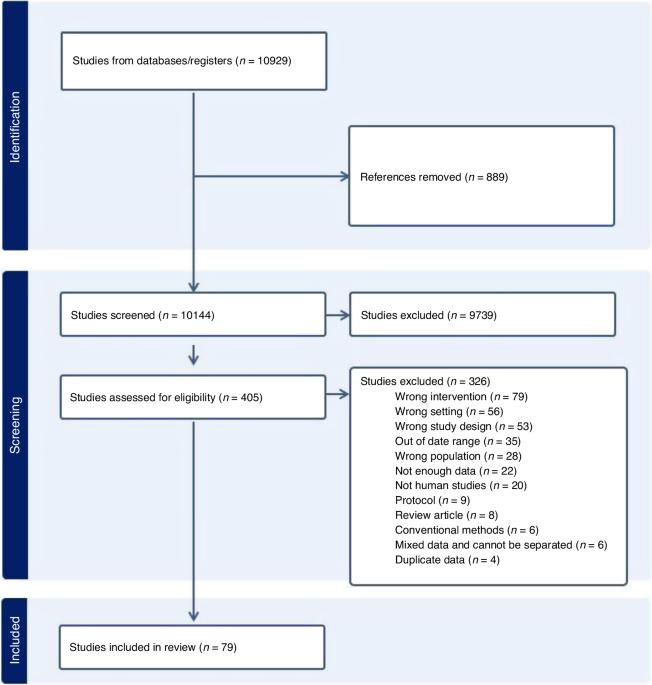
随着可穿戴生物传感器越来越多地应用于医疗机构,本综述旨在确定用于新生儿和儿科患者的可穿戴生物传感器的类型,以及如何对这些生物传感器进行临床评估。我们使用 PubMed、CINAHL、Embase、Web of Science 和 Cochrane 进行了文献检索。纳入的研究发表于 2010 年 1 月至 2024 年 2 月。研究采用描述性统计方法来显示类型、地点、临床评估方法及其结果的计数和百分比。共纳入 79 项研究。确定了 104 种可穿戴传感器和 40 种设备。最常见的生物传感器类型是光电传感器(40 个,占 38.5%)和用于测量心率的传感器(22 个,占 19.0%)。临床评估由有效性(68 人,占 86.1%)和可靠性(14 人,占 17.7%)两部分组成。只有三分之二的可穿戴设备通过了验证或报告了可接受的可靠性。大多数生物传感器研究(n = 51,64.5%)未报告任何与可穿戴生物传感器相关的并发症。目前的文献在可穿戴生物传感器设备的临床评估和安全性方面存在空白,有效性和可靠性的术语可以互换使用。目前缺乏有关并发症的全面报告,这凸显了在生物传感器医疗设备的临床评估方面制定标准化指南的必要性。影响:儿科环境中最常见的生物传感器类型是光电传感器和电传感器。只有三分之二的可穿戴设备经过验证或报告了可接受的可靠性,超过一半的生物传感器研究没有报告是否评估了与可穿戴生物传感器相关的并发症。本次综述发现了安全性和临床验证报告方面的重大差距,强调了制定标准化指南的必要性。研究结果提倡改进临床验证报告流程,以提高可穿戴生物传感器在儿科护理中的安全性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Wearable biosensors for pediatric hospitals: a scoping review
As wearable biosensors are increasingly used in healthcare settings, this review aimed to identify the types of wearable biosensors used for neonate and pediatric patients and how these biosensors were clinically evaluated. A literature search was conducted using PubMed, CINAHL, Embase, Web of Science, and Cochrane. The studies published between January 2010 and February 2024 were included. Descriptive statistics were used to present counts and percentages of types, locations, clinical evaluation methods, and their results. Seventy-nine studies were included. 104 wearable sensors and 40 devices were identified. The most common type of biosensor was optoelectrical sensors (n = 40, 38.5%), and used to measure heart rate (n = 22, 19.0%). The clinical evaluation was tested by a combination of validity (n = 68, 86.1%) and reliability (n = 14, 17.7%). Only two-thirds of the wearable devices were validated or reported acceptable reliability. The majority of the biosensor studies (n = 51, 64.5%) did not report any complications related to wearable biosensors. The current literature has gaps regarding clinical evaluation and safety of wearable biosensor devices with interchangeable use of validity and reliability terms. There is a lack of comprehensive reporting on complications, highlighting the need for standardized guidelines in the clinical evaluation of biosensor medical devices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pediatric Research
医学-小儿科
CiteScore
6.80
自引率
5.60%
发文量
473
审稿时长
3-8 weeks
期刊介绍:
Pediatric Research publishes original papers, invited reviews, and commentaries on the etiologies of children''s diseases and
disorders of development, extending from molecular biology to epidemiology. Use of model organisms and in vitro techniques
relevant to developmental biology and medicine are acceptable, as are translational human studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


