通过水性聚乳酸-聚氨酯共聚物支架重编程神经再生代谢微环境。
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
细胞新陈代谢是炎症、血管再造乃至后续组织再生的关键驱动因素,它既受信号转导的控制,也反过来受信号转导的影响。将细胞新陈代谢纳入组织工程研究,对于原位治疗修复和进一步了解宿主-生物材料在机体反应中的线索具有巨大潜力。在这项研究中,一种含有聚乳酸块的抗炎水性聚氨酯支架被用于修复神经损伤(LAx-WPU)。LAx-WPU 支架在降解过程中会释放出乳酸,随着聚乳酸块的加入,乳酸的含量会随着降解时间的延长而增加。此后,在经 LAx-WPU 降解液处理的无葡萄糖环境中,原代神经元通过单羧酸盐转运体(MCT2)吸收乳酸进行能量代谢,从而产生三磷酸腺苷(ATP)并促进神经轴突生长。将 LAx-WPU 植入大鼠脑部神经缺损修复后,28 天内大鼠脑部神经组织实现了丝状神经元伸长、快速血管化和神经组织再生,支架上的微管相关蛋白(MAP2)、β-微管蛋白(Tuj1)和血小板内皮细胞粘附分子(CD31)均呈阳性表达。结果表明,LAx-WPU支架不仅上调了ATP-ADP-AMP嘌呤代谢化合物与神经细胞的神经活性配体-受体相互作用基因、cAMP通路基因和钙通路基因之间的主要桥梁作用,而且在基因本体(GO)分析中还上调了ATP-GMP嘌呤代谢与血管生成之间的关系。进一步的反向分析表明,轴突再生受到 MCT2 的抑制,证明 LAx-WPU 促进神经修复依赖乳酸提供能量。因此,LAx-WPU支架有望在不使用任何生物活性因子的情况下,通过内在的生物材料代谢线索来调节代谢微环境,从而诱导神经再生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
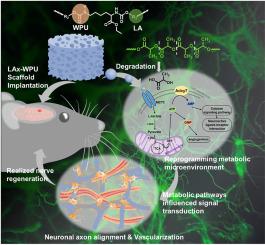
Reprogramming metabolic microenvironment for nerve regeneration via waterborne polylactic acid-polyurethane copolymer scaffolds
Cell metabolism, as the key driver of inflammation, revascularization and even subsequent tissue regeneration, is controlled by and also conversely influenced by signal transduction. Incorporation of cell metabolism into tissue engineering research holds immense potential for in-situ treatment repair and further understanding of the host-biomaterial cues in body response. In this study, an anti-inflammatory waterborne polyurethane scaffold incorporated with poly-l-lactic acid (PLLA) block was served to repair nerve injuries (LAx-WPU). Lactate was released through the degradation of LAx-WPU scaffolds, and the content increased with the addition of PLLA block over the degradation times. Thenceforth, the production of adenosine triphosphate (ATP) in primary neurons and neuronal axon growth were achieved by taking up lactate through monocarboxylate transporters (MCT2) for energy metabolism under glucose-free environment treated with LAx-WPU degradation solution. After LAx-WPU was implanted to repair brain nerve defects in rats, filamentous neurons elongation, rapid vascularization, and nerve tissue regeneration were realized up to 28 days with the positive expression of microtubule-associated protein (MAP2), β-tubulin (Tuj1), and platelet endothelial cell adhesion molecule (CD31) in the scaffolds. Results highlighted that the LAx-WPU scaffolds up-regulated not only the ATP-ADP-AMP purine metabolism compounds to mainly bridge neuroactive ligand-receptor interaction genes, cAMP pathway genes, and calcium pathway genes for neurocytes but also the ATP-GMP purine metabolism to angiogenesis in Gene Ontology (GO) analysis. Further analysis in reverse showed axonal regeneration is restrained by the inhibition of MCT2, proving LAx-WPU promoted nerve repair depended on lactate for energy. Therefore, LAx-WPU scaffolds construct an expected way to modulate the metabolic microenvironment for inducing nerve regeneration by intrinsic biomaterial metabolism cues without any bioactive factors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


