受约束的镓-镍(0)界面上的协同氢化催化作用
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
发现 3d 金属催化的独特机制对于利用这些地球上丰富的金属来替代稀缺的贵金属至关重要。受异相氢化催化剂中 Horiuti-Polanyi 机制的启发,我们描述了一种双金属分子催化剂,它可以通过配体到底物的氢化物转移机制选择性地半氢化炔烃。这模仿了已建立的异质机制,其中远距离表面结合的氢化物配体也经历了类似的反应过程。这是通过开发一种受螯合物约束的镓(I)配体实现的,该配体与镍(0)协同(可逆地)裂解 H2,生成[GaNi] 1,2-二酸酐复合物,该复合物被发现是催化过程中的静止状态。这一发现为利用非无辜的低价 13 族中心进行有效的合作催化迈出了一步,开辟了新的机理途径,可能有助于在关键的催化转化过程中使用地球上富集的金属。本文章由计算机程序翻译,如有差异,请以英文原文为准。
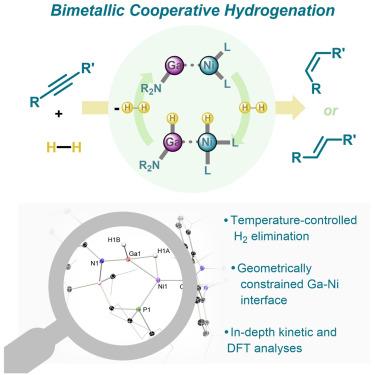
Cooperative hydrogenation catalysis at a constrained gallylene-nickel(0) interface
The discovery of unique mechanisms in 3d metal catalysis is of paramount importance in utilizing these Earth-abundant metals in place of scarce precious metals. Inspired by the Horiuti-Polanyi mechanism at play in heterogeneous hydrogenation catalysts, we describe a bimetallic molecular catalyst that can selectively semi-hydrogenate alkynes via a ligand-to-substrate hydride transfer mechanism. This mimics established heterogeneous mechanisms in which remote surface-bound hydride ligands undergo a similar reactive process. This is achieved through the development of a chelate-constrained gallium(I) ligand, which operates in concert with nickel(0) to (reversibly) cleave H2, generating a [GaNi] 1,2-dihydride complex that is found to be the resting state in the catalytic process. This discovery takes steps toward utilizing non-innocent low-valent group 13 centers in effective cooperative catalysis, opening new mechanistic pathways that may aid in employing Earth-abundant metals in key catalytic transformations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


