铁(II)、钴(II)和镍(II)与 DOTA-Tetraglycinate 的配合物,利用酰胺分子的超细位移进行 pH 值和温度成像
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
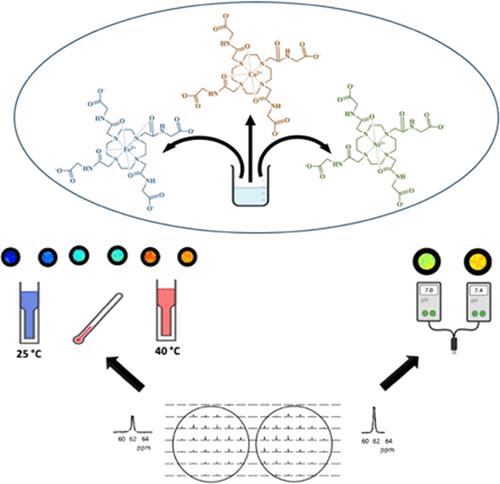
1,4,7,10-四氮杂环十二烷-1,4,7,10-四乙酸酯(DOTA4-)衍生物的顺磁配合物已显示出利用磁共振进行分子成像的潜力。与镧系金属离子(Ln3+)配位的 DOTA-tetraglycinate(DOTA-4AmC4-)可通过生物传感器位移冗余偏差成像(BIRDS)和化学交换饱和转移(CEST)分别实现 pH 值/温度传感,检测不可交换(如 -CHy,其中 3 ≥ y ≥ 1)和可交换(如 -OH 或 -NHx,其中 2 ≥ x ≥ 1)质子。在此,我们报告了二价过渡金属离子(M2+ = Fe2+、Co2+、Ni2+)与 DOTA-4AmC4- 的顺磁配合物,这些配合物具有独特的酰胺质子(-NH)分子,可用于 pH 值/温度传感。晶体学数据显示,DOTA-4AmC4- 通过氧和氮供体原子与 M2+ 配位,配位数从 Fe(II)DOTA-4AmC2- 的 8 个配位、Co(II)DOTA-4AmC2- 的 7 个配位和 Ni(II)DOTA-4AmC2- 的 6 个配位不等。M(II)DOTA-4AmC2- 中的 -CHy 质子显示出适度的 pH 值/温度敏感性,但 -NH 质子显示出更高的强度,表明其具有突出的 BIRDS 特性。Ni(II)DOTA-4AmC2- 的 pH 灵敏度最高(1.42 ppm/pH),其次是 Co(II)DOTA-4AmC2- (0.21 ppm/pH)和 Fe(II)DOTA-4AmC2- (0.16 ppm/pH),而温度灵敏度相当(分别为 0.22、0.13 和 0.17 ppm/°C)。与 Ln(III)DOTA-4AmC- 相比,M(II)DOTA-4AmC2- 中 -NH 的 CEST 图像对比度要弱得多。鉴于 Ni(II)DOTA-4AmC2- 具有较高的 pH 值灵敏度和较低的细胞毒性,因此有望用于临床前基于 BIRDS 的 pH 值成像。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Complexes of Iron(II), Cobalt(II), and Nickel(II) with DOTA-Tetraglycinate for pH and Temperature Imaging Using Hyperfine Shifts of an Amide Moiety
Paramagnetic complexes of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate (DOTA4–) derivatives have shown potential for molecular imaging with magnetic resonance. DOTA-tetraglycinate (DOTA-4AmC4–) coordinated with lanthanide metal ions (Ln3+) demonstrates pH/temperature sensing with Biosensor Imaging of Redundant Deviation in Shifts (BIRDS) and Chemical Exchange Saturation Transfer (CEST), respectively, detecting nonexchangeable (e.g., −CHy, where 3 ≥ y ≥ 1) and exchangeable (e.g., −OH or –NHx, where 2 ≥ x ≥ 1) protons. Herein, we report paramagnetic complexes of divalent transition-metal ions (M2+ = Fe2+, Co2+, Ni2+) with DOTA-4AmC4– that endow a unique amide proton (−NH) moiety for pH/temperature sensing. Crystallographic data reveal that DOTA-4AmC4– coordinates with M2+ through oxygen and nitrogen donor atoms, ranging in coordination numbers from 8-coordinate in Fe(II)DOTA-4AmC2–, 7-coordinate in Co(II)DOTA-4AmC2–, and 6-coordinate in Ni(II)DOTA-4AmC2–. The −CHy protons in M(II)DOTA-4AmC2– displayed modest pH/temperature sensitivities, but –NH protons exhibited higher intensity, suggesting prominent BIRDS properties. The pH sensitivity was the highest for Ni(II)DOTA-4AmC2– (1.42 ppm/pH), followed by Co(II)DOTA-4AmC2– (0.21 ppm/pH) and Fe(II)DOTA-4AmC2– (0.16 ppm/pH), whereas temperature sensitivities were comparable (i.e., 0.22, 0.13, and 0.17 ppm/°C, respectively). The CEST image contrast for –NH in M(II)DOTA-4AmC2– was much weaker compared to that of Ln(III)DOTA-4AmC–. Given its high pH sensitivity and low cytotoxicity, Ni(II)DOTA-4AmC2– shows promise for use in preclinical BIRDS-based pH imaging.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


