开发用于氨和二氧化碳捕集及转化的多功能类胆碱离子溶剂
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本研究开发了两种离子溶剂,使用胆碱(ChCl)家族的氯化物:带有 2-羟乙基的铵盐、二甲基-二(2-羟乙基)氯化铵(1)和甲基-三(2-羟乙基)氯化铵(2)作为氢键受体(HBA),甘油(Gly)作为氢键供体(HBD),摩尔比为 1:2。在温度为 303.2 至 333.2 K 和压力为 100 至 813 kPa 的条件下,测量了氨和二氧化碳在这些溶剂中的溶解度,以及二氧化碳在甘油(ChCl : Gly = 1:2)和纯甘油中的溶解度。由此确定了吸收过程中的亨利定律常数和热力学函数的变化。我们发现,与只含有一个 2- 羟乙基取代基的 HBA 的甘精相比,氨和二氧化碳在这些由 2- 羟乙基接枝氢键受体(HBA)制成的离子溶剂中的溶解度随着 2- 羟乙基的数量增加而增加。开发的流体还在二氧化碳与环氧氯丙烷(ECH)的环加成反应中表现出催化活性,生成碳酸氯丙烯酯(PC-Cl)。在 100 °C、650 kPa 和 2 mol % 催化剂浓度的反应条件下,ECH 在 6 小时内实现了 95% 的转化率。此外,还测量了纯液体和气体饱和液体的密度(ρ)和粘度(η),从而完成了离子溶剂的全面表征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
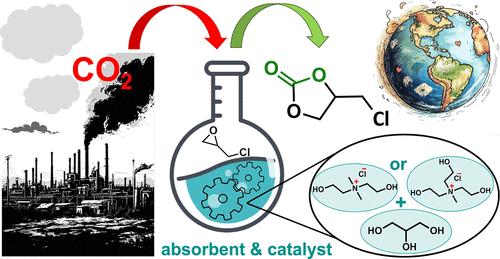
Development of Multifunctional Choline-Like Ionic Solvents for Ammonia and Carbon Dioxide Capture and Transformation of Latter
This study developed two ionic solvents using chlorides from the choline (ChCl) family: ammonium salts with 2-hydroxyethyl groups, dimethyl-di(2-hydroxyethyl)-ammonium chloride (1) and methyl-tri(2-hydroxyethyl)-ammonium chloride (2) as the hydrogen bond acceptor (HBA) and glycerol (Gly) as the hydrogen bond donor (HBD) in a molar ratio of 1:2. The solubility of ammonia and carbon dioxide in these solvents and also solubility of carbon dioxide in glycelin (ChCl : Gly = 1:2) and pure glycerol were measured at temperatures ranging from 303.2 to 333.2 K and pressures from 100 to 813 kPa. From this, Henry’s law constants and the changes in thermodynamic functions during the absorption process were determined. We show that solubility of ammonia and carbon dioxide in these ionic solvents made from 2-hydroxyethyl-grafted hydrogen bond acceptors (HBAs) increases with the number of 2-hydroxyethyl groups compared to the glycelin comprising HBA with one 2-hydroxyethyl substituent only. Developed fluids also demonstrated catalytic activity in the cycloaddition of CO2 to epichlorohydrin (ECH), producing chloropropylene carbonate (PC-Cl). Under reaction conditions of 100 °C, 650 kPa, and 2 mol % catalyst concentration, a 95% conversion of ECH was achieved within 6 h. The effect of various reaction conditions on the ECH conversion and PC-Cl yield was also investigated. Additionally, the densities (ρ) and viscosities (η) of both pure and gas-saturated fluids were measured, completing the comprehensive characterization of the ionic solvents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


