炔酮促进络氨酸与 C-和 N-亲核物的脱氨基偶联反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
络氨酸与亲核物的脱氨基偶联是结构多样化的一种通用方法,但往往涉及非纯净条件和/或试剂。在此,我们开发了一种新的乙炔试剂 2,用于炔苷的 C-N 键活化及其与以 C 和 N 为中心的亲核物的原位偶联。使用新的酸/碱和氧化还原中性炔酮试剂 2,偶联反应进行得非常顺利,例如合成了几种吲哚-3-基甲基衍生物,包括新的吲哚-苯并二氮杂卓和吲哚-腙共轭物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
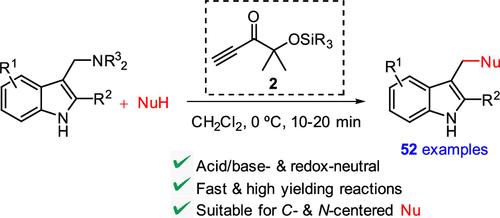
Ynone Promoted Deaminative Coupling of Gramines with C- and N-Nucleophiles
The deaminative coupling of gramines with nucleophiles represents a versatile approach for structure diversification, but often involves non innocent conditions and/or reagents. Here a new acetylenic reagent 2 is developed for the C–N bond activation of gramines and their in situ coupling with C- and N-centered nucleophiles. Using the new acid/base- and redox-neutral ynone reagent 2 the coupling reactions proceed exceedingly as exemplified by the synthesis of several indol-3-ylmethyl derivatives, including new indole-benzodiazepine and indole-hydrazone conjugates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


