基于调节炎症和免疫平衡的 MnNi@PVP 纳米酶用于治疗炎症性肠病
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
纳米酶具有显著的抗氧化能力,是治疗溃疡性结肠炎(UC)的有效方法。本研究通过高温热解和氯化钠模板法合成了 MnNi@PVP 纳米酶,该纳米酶具有多酶活性,包括谷胱甘肽过氧化物酶(GPx)、超氧化物歧化酶(SOD)和过氧化氢酶(CAT)。本研究探讨了 MnNi@PVP 对右旋糖酐硫酸钠(DSS)引起的结肠炎的治疗效果。研究结果表明,MnNi@PVP 显著降低了疾病活动指数(DAI)评分,其中包括体重下降、结肠缩短和组织病理学变化。MnNi@PVP 通过使 TLR4 通路和巨噬细胞失活,在解决氧化损伤和抑制肿瘤坏死因子 (TNF-α)、诱导型一氧化氮合酶 (iNOS) 和白细胞介素 (IL)-6 等促炎标志物水平方面显示出有效性。此外,锰镍(MnNi)@PVP 还能修复紧密连接蛋白(occludin 和 ZO-1),恢复肠道屏障。转录组测序表明,MnNi@PVP 可调节炎症因子表达途径和免疫过程。此外,带负电荷的 MnNi@PVP 可通过静电相互作用选择性地与发炎的结肠组织结合,使其在肠道炎症部位具有靶向修复能力。锰镍(MnNi)@PVP 具有本节研究的活性氧和氮物种(RONS)清除能力,有望为有针对性地修复肠道功能奠定基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
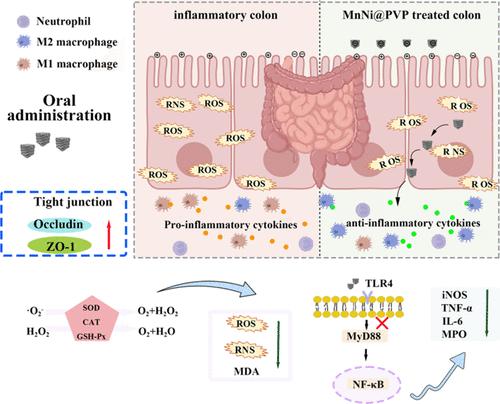
MnNi@PVP Nanoenzyme Based on Regulating Inflammation and Immune Homeostasis for the Therapy of Inflammatory Bowel Disease
Nanozymes exhibiting remarkable antioxidant capabilities represent an effective therapeutic approach for ulcerative colitis (UC). This study synthesized the MnNi@PVP nanoenzyme through high-temperature pyrolysis and the NaCl template method, which exhibited multienzyme activity comprising glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT). This study investigated the therapeutic effect of MnNi@PVP on colitis caused by sodium dextran sulfate (DSS). The findings indicated that MnNi@PVP notably decreased the disease activity index (DAI) score, which encompasses weight loss, colon shortening, and histopathological changes. MnNi@PVP showed effectiveness in addressing oxidative damage and suppressing the levels of proinflammatory markers, such as tumor necrosis factor (TNF-α), inducible nitric oxide synthase (iNOS), and interleukin (IL)-6, by inactivating the TLR4 pathway and macrophages. In addition, MnNi@PVP demonstrated the ability to repair tight junction proteins (occludin and ZO-1) and restore the intestinal barrier. The transcriptome sequencing demonstrated that MnNi@PVP could regulate inflammatory factor expression pathways and immune processes. Additionally, negatively charged MnNi@PVP can selectively bind to inflamed colonic tissues through electrostatic interactions, endowing it with targeted reparative capabilities at the location of intestinal inflammation. The MnNi@PVP, which possesses the reactive oxygen and nitrogen species (RONS) clearance capability examined in this section, is expected to provide the basis for the targeted repair of intestinal function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


