作为治疗 MSI 肿瘤的 WRN 抑制剂的三唑并嘧啶衍生物的设计、合成和结构-活性关系研究
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
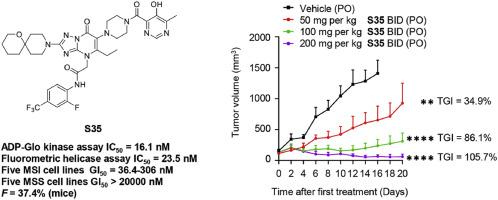
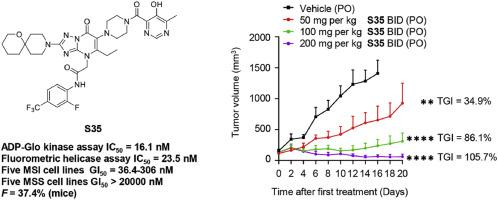
维尔纳综合征 RecQ 螺旋酶(WRN)是 RecQ 螺旋酶家族的成员,最近被确定为微卫星不稳定性(MSI)肿瘤的合成致死靶点。三唑并嘧啶化合物 HRO761 是第一个进入临床试验的 WRN 抑制剂,但对这一支架的研究仍然有限。在此,我们设计了一系列衍生物来系统研究三唑并嘧啶支架的结构-活性关系(SAR),最终发现了化合物 S35。S35 具有出色的 WRN 螺旋酶抑制活性(ADP-Glo 激酶测定 IC50 = 16.1 nM,荧光螺旋酶测定 IC50 = 23.5 nM)。此外,S35 还表现出极佳的细胞选择性,对多种 MSI 细胞株具有抗增殖活性(GI50 = 36.4-306 nM),而对多种微卫星稳定性(MSS)细胞株的 GI50 值大于 20000 nM。此外,我们还观察到化合物 S35 在 MSI 细胞中诱导 DNA 损伤并导致 G2/M 细胞周期停滞,而在 MSS 细胞中则不会发生这种情况。S35 表现出良好的口服药物动力学特性,口服给药可在 SW48 异种移植模型中产生剂量依赖性肿瘤生长抑制作用。这些发现为开发用于治疗MSI肿瘤的WRN抑制剂提供了良好的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design, synthesis, and structure−activity relationship studies of triazolo-pyrimidine derivatives as WRN inhibitors for the treatment of MSI tumors
Werner syndrome RecQ helicase (WRN), a member of the RecQ helicase family, has recently been identified as a synthetic lethal target in microsatellite instability (MSI) tumors. The triazolo-pyrimidine compound HRO761 is the first WRN inhibitor to enter clinical trials, but research on this scaffold remains limited. Here, we designed a series of derivatives to systematically study the structure-activity relationship (SAR) of triazolo-pyrimidine scaffolds, leading to the discovery of compound S35. S35 exhibited excellent WRN helicase inhibitory activity (ADP-Glo kinase assay IC50 = 16.1 nM, fluorometric helicase assay IC50 = 23.5 nM). Additionally, S35 exhibited excellent cellular selectivity, with antiproliferative activity against multiple MSI cell lines (GI50 = 36.4−306 nM), while the GI50 values for multiple microsatellite stability (MSS) cell lines were greater than 20,000 nM. Furthermore, we observed that compound S35 induced DNA damage and caused G2/M cell cycle arrest in MSI cells, which did not occur in MSS cells. S35 demonstrated favorable oral pharmacokinetic properties, with oral administration resulting in dose-dependent tumor growth inhibition in the SW48 xenograft model. These findings provide a promising outlook for the development of WRN inhibitors for the treatment of MSI tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


