发现吩嗪衍生物作为一类新的非经典铁中毒抑制剂,并对小鼠肝损伤模型进行疗效评估
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
铁变性是一种铁依赖性调节细胞死亡,与多种疾病的发生和发展有关。铁突变抑制剂被认为是治疗这些相关疾病的潜在药物。然而,目前大多数可用的铁蛋白沉积抑制剂都是抗氧化剂或铁螯合剂(称为经典铁蛋白沉积抑制剂),在临床使用过程中可能存在潜在的副作用风险。在此,我们报告了吩嗪衍生物作为一类新的非经典铁氧化酶抑制剂的发现。通过对这些系列化合物的结构-活性关系研究,我们发现了活性最高的化合物 13l,其 EC50 值为 0.0007 μM。从机理上讲,13l 可抑制 NCOA4 介导的噬铁蛋白作用,从而保护细胞免受铁变态反应的影响。值得注意的是,在对乙酰氨基酚诱导的急性肝损伤模型中,13l 表现出了很好的治疗效果。总之,本文报道的这种化合物可能是一种很有前景的先导化合物,可用于靶向铁蛋白沉积的药物研发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
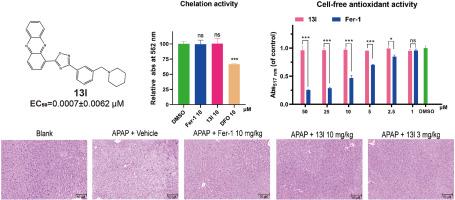
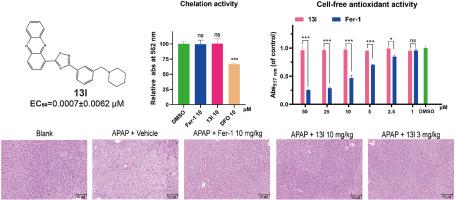
Discovery of phenazine derivatives as a new class of non-classical ferroptosis inhibitors and efficacy evaluation on a mouse model of liver injury
Ferroptosis is an iron-dependent regulated cell death, which has been implicated in the onset and progression of numerous diseases. Ferroptosis inhibitors are thought as potential agents for treating these related diseases. However, the majority of currently available ferroptosis inhibitors are antioxidants or iron chelators (called classical ferroptosis inhibitors), which might have potential risks of side effects during clinical use. Herein, we report the discovery of phenazine derivatives as a new class of non-classical ferroptosis inhibitors. Structure-activity relationship of these series compounds led to the discovery of the most active compound 13l with an EC50 value of 0.0007 μM. Mechanistically, 13l could inhibit NCOA4-mediated ferritinophagy, hence protecting cells from ferroptosis. Notably, in the acetaminophen-induced acute liver injury model, 13l showed an excellent therapeutic effect. Overall, this compound reported here could be a promising lead compound for drug discovery targeting ferroptosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


