鉴定具有流行单原子修饰的生物活性化合物:综合分析及对化合物设计的影响
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
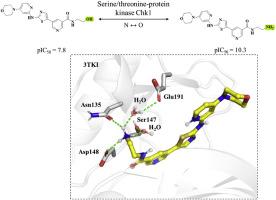
ChEMBL 等公共数据库提供的大量生物活性数据有助于进行深入的结构-活性关系(SAR)分析,这对于了解分子修饰对生物活性的影响至关重要。SAR 分析的核心策略是评估分子相似性。目前已开发出几种药物化学家偏爱的方法,可有效地大规模捕获结构相关的化合物。单原子修饰(SAMs)作为一种流行的分子编辑策略,被广泛应用于 "命中先导"(hit-to-lead)和 "先导优化"(lead optimization)过程中,我们之前介绍了四种单原子修饰(SAMs),并对其在化合物设计中的应用进行了系统分析。在本研究中,我们扩大了分析范围,涵盖了 10 种常见的单原子修饰,包括碳-氮(N↔C)、O↔C、N↔O、S↔O 以及更简单的修饰,如 OH↔H、CH3↔H 和卤素-氢(F、Cl、Br、I ↔ H)交换。利用来自 ChEMBL(34 版)的高置信度生物活性数据,我们建立了迄今为止最大的 SAM 对数据集,包括 374,979 对。在对这些 SAM 类型在药物化学中的出现频率进行评估后,我们重点研究了 SAM 诱导的活性悬崖(AC),得出了 7,400 多个涉及 SAM 的 AC,大大扩展了目前与单原子变化相关的 AC 的知识库。此外,在实验数据的支持下,这些 AC 的结构分析为了解单原子修饰在调节化合物活性方面的作用提供了重要见解,为在药物开发过程中基于结构优化分子特性提供了实用指导。因此,我们将开放所有已鉴定的 ACs 及其相关结构信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Identification of bioactive compounds with popular single-atom modifications: Comprehensive analysis and implications for compound design
The extensive bioactivity data available in public databases, such as ChEMBL, has facilitated in-depth structure-activity relationship (SAR) analysis, which are essential for understanding the impact of molecular modifications on biological activity in a comprehensive manner. A central strategy in SAR analysis is the assessment of molecular similarity. Several approaches preferred by medicinal chemists have been developed to efficiently capture structurally related compounds on a large scale. Represented as a popular molecular editing strategy in hit-to-lead and lead optimization processes, we previously introduced four types of single-atom modifications (SAMs) as chemical similarity criterion and conducted a systematic analysis of their application in compound design. In this study, we expanded the analysis to cover 10 common SAMs, including carbon-nitrogen (N↔C), O↔C, N↔O, S↔O, as well as simpler modifications such as OH↔H, CH3↔H, and halogen-hydrogen (F, Cl, Br, I↔H) exchanges. Leveraging high-confidence bioactivity data from ChEMBL (version 34), we assembled a comprehensive dataset comprising 374,979 SAM pairs. Following an evaluation of the frequency of these SAM types in medicinal chemistry efforts, we focused on SAM-induced activity cliffs (ACs), yielding over 7400 ACs, substantially expanding the current knowledgebase of ACs associated with single-atom changes. Furthermore, structural analysis of these ACs, supported by experimental data, provides critical insights into the role of single-atom modifications in modulating compound activity, offering practical guidance for the structure-based optimization of molecular properties in drug development. As a result, we are providing open access to all identified ACs along with their associated structural information.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


