逆转融合内酯合成中酶促动态不对称转化的对映选择性
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
将一种酮还原酶合理地设计成立体互补变体,以控制带有连续立体中心的笨重手性分子的立体选择性,是非常理想和具有挑战性的。在此,我们报告了通过对位于酶袋中的两个关键残基(Y188 和 H145)进行定向诱变,对来自 Chryseobacterium sp.值得注意的是,在该系统中观察到了动力学解析不对称还原(KR-AR)和动态动力学不对称转化(DyKAT)。在 KR-AR 过程中,ChKRED20 变体只将 (R)- 或 (S)- 酮酯转化为相应的对映体和非对映富集的 (R,S)- 或 (S,R)- 顺式内酯,并提供剩余的 (S)- 或 (R)- 酮酯。相反,在 DyKAT 过程中,底物的非反应构型通过酶中的质子化-质子化作用进行有效的烯醇化平衡。此外,还进行了计算研究,以深入了解立体选择性和对映体选择性的起源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
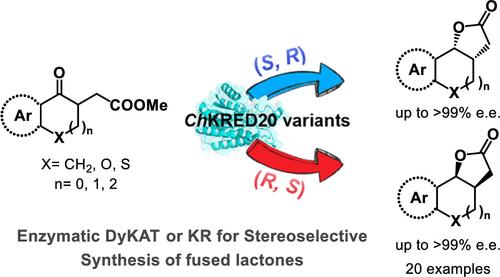
Reversing the Enantioselectivity of Enzymatic Dynamic Kinetic Asymmetric Transformations in the Synthesis of Fused Lactones
The rational design of one ketoreductase into stereocomplementary variants for controlling the stereoselectivity of bulky chiral molecules bearing contiguous stereocenters is highly desirable and challenging. Herein, we report protein engineering of ketoreductase from Chryseobacterium sp. CA49 (ChKRED20) through targeted mutagenesis of only two key residues (Y188 and H145) located in the enzyme pocket, achieving the precise stereocontrol over the synthesis of tricyclic fused lactones (highest reversing enantioselectivity from >99:1 e.r. to <1:99 e.r.). Notably, both kinetic resolution asymmetric reduction (KR-AR) and dynamic kinetic asymmetric transformation (DyKAT) were observed in this system. In the KR-AR process, ChKRED20 variants exclusively convert (R)- or (S)-keto esters to corresponding enantio- and diastereoenriched (R,S)- or (S,R)-cis-lactones and deliver leftover (S)- or (R)-keto esters. On the contrary, in the DyKAT process, unreactive configurations of substrates undergo efficient equilibration via an enolization through protonation–deprotonation in enzymes. Computational studies are also conducted to get insight into the origin of stereoselectivity and enantioselectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


