调整二氧化 CeO2 支承二氧化锡上的氧空位以实现高效的二氧化碳电还原
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
在中性或碱性水溶液中,H2O 作为质子源,有利于 CO2 的电还原,但却存在动力学迟缓的问题。在此,我们合成了一系列具有不同氧空位(Ov)浓度的 SnO2-CeO2 催化剂,调节 H2O 的解离,使其与 CO2 还原同步。Ov浓度适中的最佳 SnO2-CeO2 催化剂的甲酸法拉第效率接近 93%,在 100 mA/cm2 的电流密度下可维持 46 小时以上。DFT 计算和原位衰减全反射-傅立叶变换红外光谱(ATR-FTIR)证实,较低 Ov 浓度的催化剂会导致 H2O 离解较弱,从而提高 *OCHO 生成的能垒;而较高的 Ov 浓度则会导致质子过多,加剧氢进化反应(HER)。这项研究强调了 Ov 浓度在决定支撑电催化剂水解离能力方面的重要性,为开发更高效的电催化剂提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
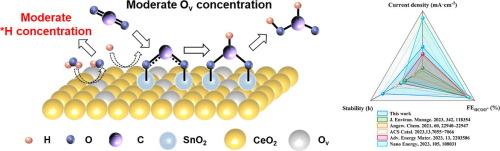
Tuning oxygen vacancy for efficient CO2 electroreduction over CeO2 supported SnO2
CO2 electroreduction is favorable in neutral or alkaline aqueous solutions, where H2O serves as the proton source, suffering from sluggish dynamics. Herein, we synthesize a series of SnO2-CeO2 with different oxygen vacancy (Ov) concentration, regulating the H2O dissociation, to synchronize with the CO2 reduction. The optimal SnO2-CeO2 catalyst, with a moderate Ov concentration, exhibits a formate Faradic efficiency of nearly 93% and maintains for more than 46 h at a current density of 100 mA/cm2. The catalyst with lower Ov concentration results in weak H2O dissociation, thus enhancing the energy barrier of *OCHO generation, while higher Ov concentration leads to excessive proton, exacerbating the hydrogen evolution reaction (HER), as supported by DFT calculation and in situ attenuated total reflection-Fourier transform infrared spectra (ATR-FTIR). This study underscores the significance of Ov concentration in determining the ability of water dissociation over supported electrocatalysts, providing valuable insights for the development of more efficient electrocatalyst.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


